Abstract
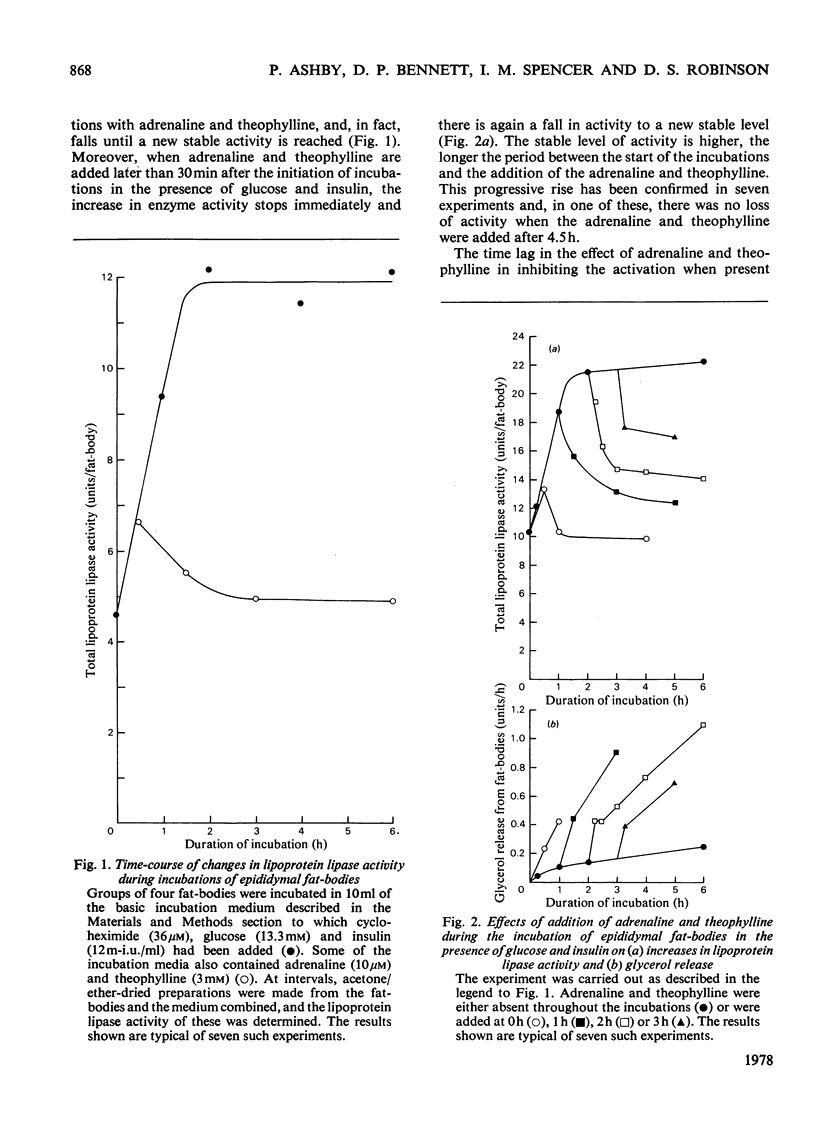
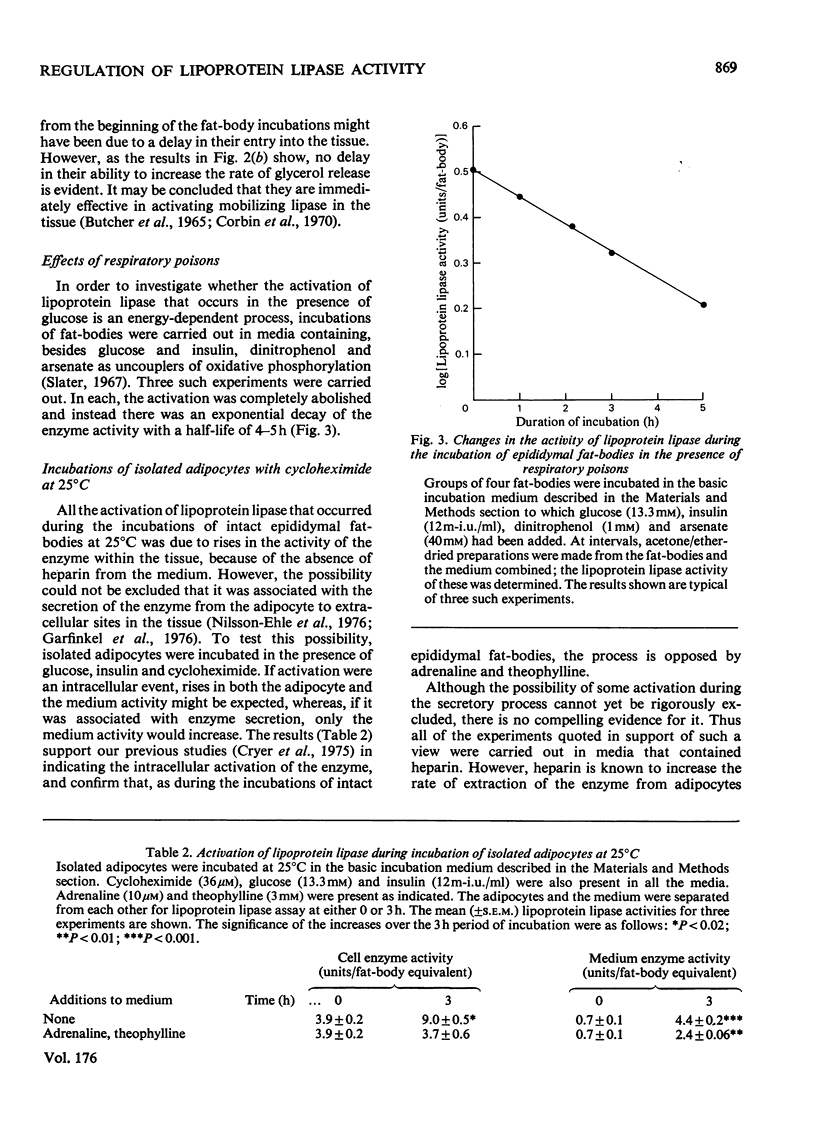
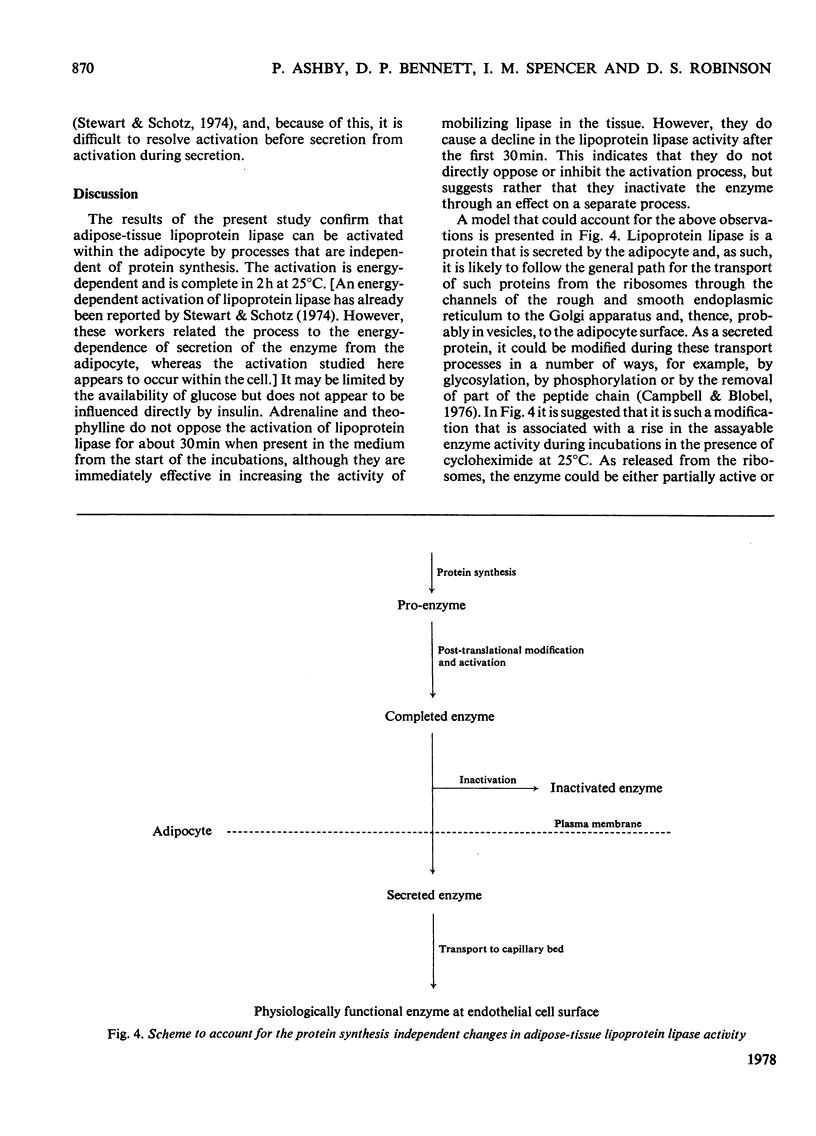
Changes in adipose-tissue lipoprotein lipase activity that are independent of protein synthesis were investigated in an incubation system in vitro. Under appropriate conditions at 25 degrees C a progressive increase in the enzyme activity occurs that is energy-dependent. Part of the enzyme is rapidly inactivated when the tissue is incubated with adrenaline or adrenaline plus theophylline. The mechanism of this inactivation appears to be distinct from, and to follow, the activation of the enzyme. A hypothesis is presented to account for the results in terms of an activation of the enzyme during obligatory post-translational processing and a catecholamine-regulated inactivation of the enzyme as an alternative to secretion from the adipocyte.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby P., Tolson A. M., Robinson D. S. The heterogeneity of the lipoprotein lipase of rat epididymal adipose tissue. Biochem J. 1978 May 1;171(2):305–311. doi: 10.1042/bj1710305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Ehnholm C., Steinberg D., Brown W. V. Purification and characterization of lipoprotein lipase from pig adipose tissue. J Biol Chem. 1974 Apr 10;249(7):2220–2227. [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Campbell P. N., Blobel G. The role of organelles in the chemical modification of the primary translation products of secretory proteins. FEBS Lett. 1976 Dec 31;72(2):215–226. doi: 10.1016/0014-5793(76)80973-8. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M., Walsh D. A., Krebs E. G. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3',5'- monophosphate-stimulated protein kinase. J Biol Chem. 1970 Sep 25;245(18):4849–4851. [PubMed] [Google Scholar]
- Cryer A., Davies P., Williams E. R., Robinson D. S. The clearing-factor lipase activity of isolated fat-cells. Biochem J. 1975 Feb;146(2):481–488. doi: 10.1042/bj1460481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A., Foster B., Wing D. R., Robinson D. S. The effect of cycloheximide on adipose-tissue clearing-factor lipase. Biochem J. 1973 Apr;132(4):833–836. doi: 10.1042/bj1320833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A., Riley S. E., Williams E. R., Robinson D. S. Effect of nutritional status on rat adipose tissue, muscle and post-heparin plasma clearing factor lipase activities: their relationship to triglyceride fatty acid uptake by fat-cells and to plasma insulin concentrations. Clin Sci Mol Med. 1976 Mar;50(3):213–221. doi: 10.1042/cs0500213. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Robinson D. S. Clearing-factor lipase in adipose tissue. Distinction of different states of the enzyme and the possible role of the fat cell in the maintenance of tissue activity. Biochem J. 1969 Apr;112(2):203–209. doi: 10.1042/bj1120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Cryer A., Robinson D. S. Hormonal control of adipose tissue clearing factor lipase activity. FEBS Lett. 1974 Sep 1;45(1):271–275. doi: 10.1016/0014-5793(74)80860-4. [DOI] [PubMed] [Google Scholar]
- García-Sáinz J. A., Piña E., Chagoya de Sánchez V. Stimulatory action of cycloheximide on glucose metabolism in the rat epididymal fat pad. J Lipid Res. 1977 Jan;18(1):93–98. [PubMed] [Google Scholar]
- Garfinkel A. G., Nilsson-ehle P., Schotz M. C. Regulation of lipoprotein lipase. Induction by insulin. Biochim Biophys Acta. 1976 Feb 23;424(2):264–273. doi: 10.1016/0005-2760(76)90194-6. [DOI] [PubMed] [Google Scholar]
- Garfinkel A. S., Schotz M. C. Separation of molecular species of lipoprotein lipase from adipose tissue. J Lipid Res. 1972 Jan;13(1):63–68. [PubMed] [Google Scholar]
- Garfinkel A. S., Schotz M. C. Sequential induction of two species of lipoprotein lipase. Biochim Biophys Acta. 1973 Apr 13;306(1):128–133. doi: 10.1016/0005-2760(73)90216-6. [DOI] [PubMed] [Google Scholar]
- HAGEN J. H., HAGEN P. B. An enzymic method for the estimation of glycerol in blood and its use to determine the effect of noradrenaline on the concentration of glycerol in blood. Can J Biochem Physiol. 1962 Aug;40:1129–1139. [PubMed] [Google Scholar]
- Hawkins R. A., Alberti K. G., Houghton C. R., Williamson D. H., Krebs H. A. The effect of acetoacetate on plasma insulin concentration. Biochem J. 1971 Nov;125(2):541–544. doi: 10.1042/bj1250541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- Moskowitz J., Fain J. N. Stimulation by growth hormone and dexamethasone of labeled cyclic adenosine 3',5'-monophosphate accumulation by white fat cells. J Biol Chem. 1970 Mar 10;245(5):1101–1107. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Intra- and extracellular forms of lipoprotein lipase in adipose tissue. Biochim Biophys Acta. 1976 Apr 22;431(1):147–156. doi: 10.1016/0005-2760(76)90269-1. [DOI] [PubMed] [Google Scholar]
- Numa S. Regulation of fatty-acid synthesis in higher animals. Ergeb Physiol. 1974;69(0):54–96. [PubMed] [Google Scholar]
- Salaman M. R., Robinson D. S. Clearing-factor lipase in adipose tissue. A medium in which the enzyme activity of tissue from starved rats increases in vitro. Biochem J. 1966 Jun;99(3):640–647. doi: 10.1042/bj0990640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotz M. C., Garfinkel A. S. Effect of nutrition on species of lipoprotein lipase. Biochim Biophys Acta. 1972 Aug 11;270(4):472–478. doi: 10.1016/0005-2760(72)90112-9. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Blanchette-Mackie E. J., Smith L. C. Role of capillary endothelium in the clearance of chylomicrons. A model for lipid transport from blood by lateral diffusion in cell membranes. Circ Res. 1976 Aug;39(2):149–162. doi: 10.1161/01.res.39.2.149. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differentiation. Interactions of insulin, prolactin and cortisol in controlling the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1976 Feb 15;154(2):359–370. doi: 10.1042/bj1540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Khoo J. C. Hormone-sensitive lipase of adipose tissue. Fed Proc. 1977 Jun;36(7):1986–1990. [PubMed] [Google Scholar]
- Stewart J. E., Schotz M. C. Release of lipoprotein lipase activity from isolated fat cells. II. Effect of heparin. J Biol Chem. 1974 Feb 10;249(3):904–907. [PubMed] [Google Scholar]
- Stewart J. E., Schotz M. C. Studies on release of lipoprotein lipase activity from fat cells. J Biol Chem. 1971 Sep 25;246(18):5749–5753. [PubMed] [Google Scholar]
- Wing D. R., Fielding C. J., Robinson D. S. The effect of cycloheximide on tissue clearing-factor lipase activity. Biochem J. 1967 Sep;104(3):45C–46C. doi: 10.1042/bj1040045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing D. R., Robinson D. S. Clearing-factor lipase in adipose tissue. A possible role of adenosine 3',5'-(cyclic)-monophosphate in the regulation of its activity. Biochem J. 1968 Oct;109(5):841–849. doi: 10.1042/bj1090841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing D. R., Robinson D. S. Clearing-factor lipase in adipose tissue. Studies with puromycin and actinomycin. Biochem J. 1968 Feb;106(3):667–676. doi: 10.1042/bj1060667. [DOI] [PMC free article] [PubMed] [Google Scholar]


