Abstract
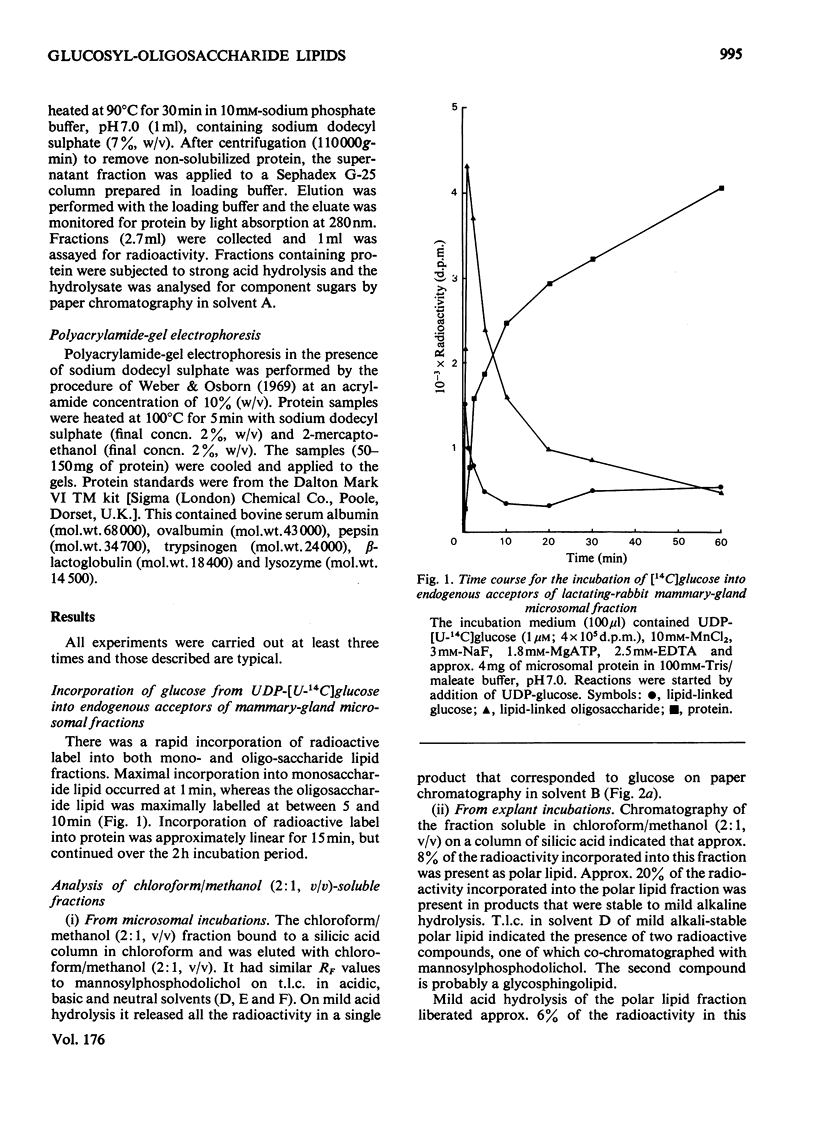
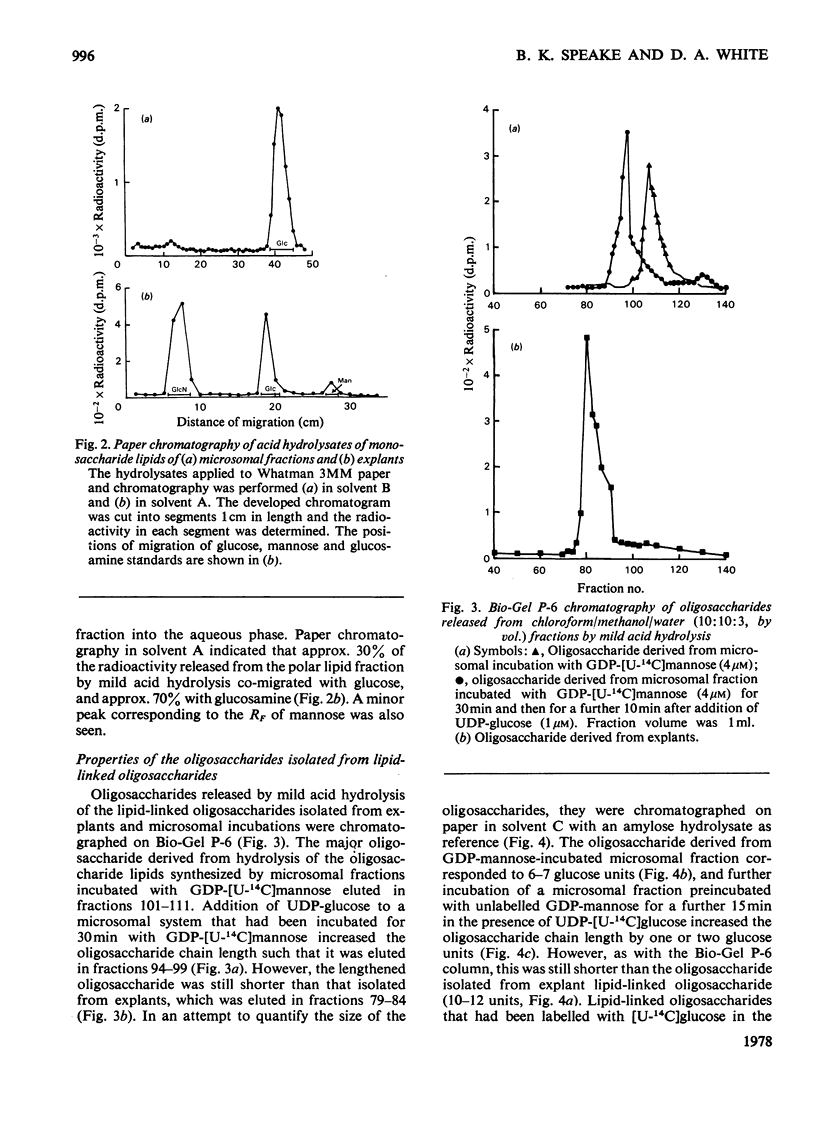
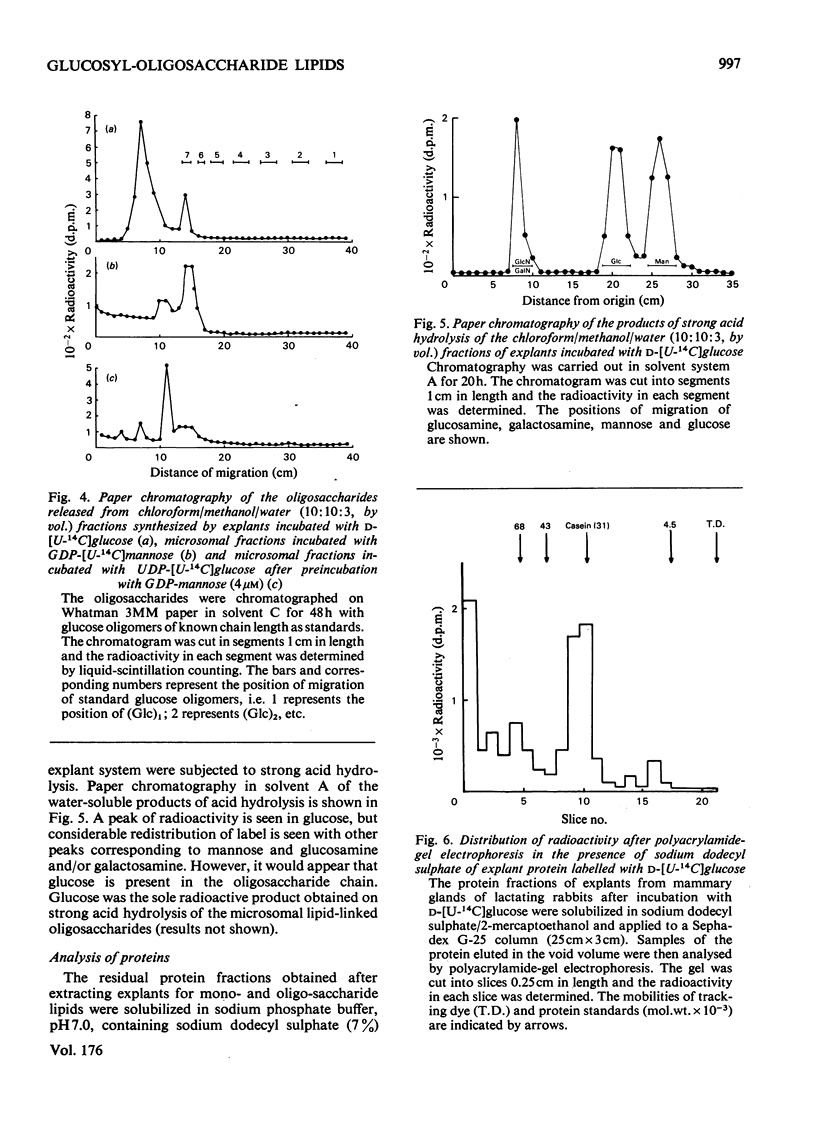
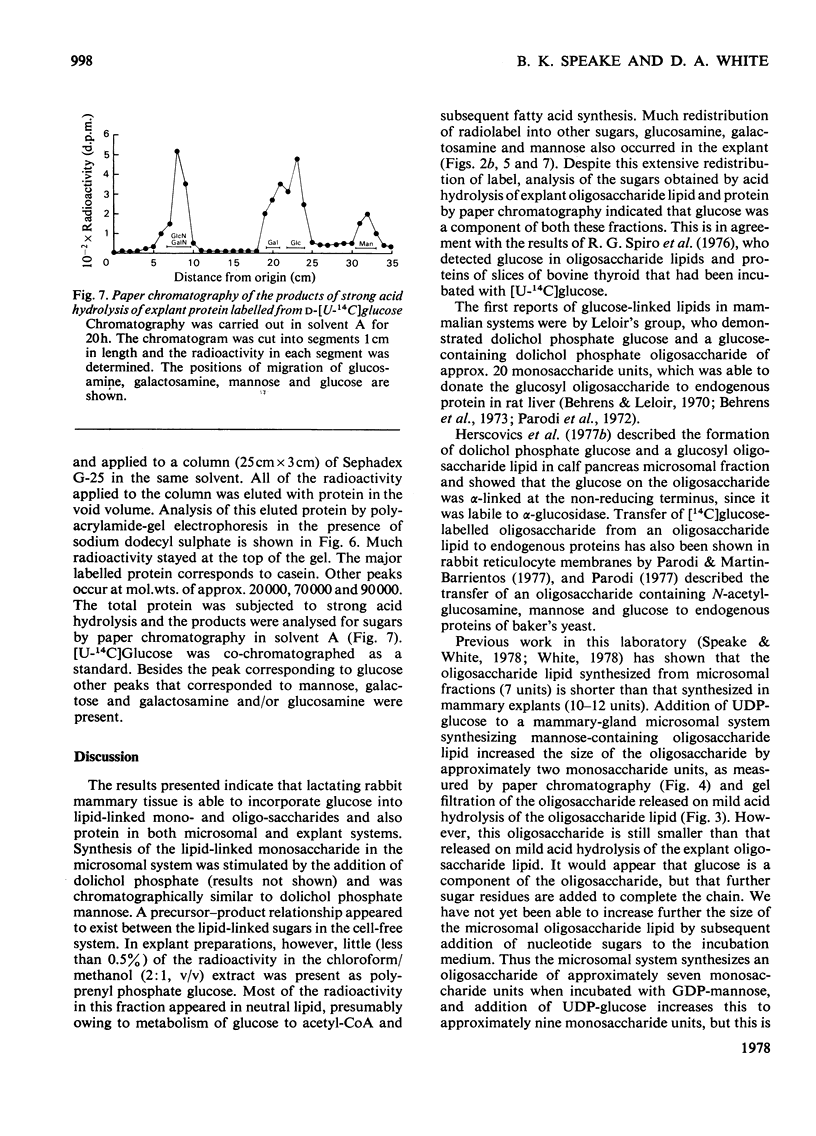
1. Microsomal fractions of lactating rabbit mammary gland incubated with UDP-glucose formed lipid-linked mono- and oligo-saccharides. The lipid-linked monosaccharide had chromatographic properties similar to those of dolichol phosphate mannose and yielded glucose on acid hydrolysis. 2. Incubation of the microsomal fraction with GDP-[U14C]-mannose yielded an oligosaccharide lipid of approximately seven monosaccharide units. Further incubation with UDP-glucose increased the size of the oligosaccharide by approximately two units. 3. Explants of lactating rabbit mammary gland incorporated [U-14C]glucose into both lipid-linked mono- and oligo-saccharides. The oligosaccharide lipid was of approx. 11 monosaccharide units. 4. Considerable redistribution of radioactive label occurred in the explant system, and radioactively labelled glucosamine and mannose, as well as glucose, were detected on acid hydrolysis of the oligosaccharide lipid. 5. Glucose was also detected in the acid hydrolysate of explant proteins. Radioactive glucosamine, galactosamine, galactose and mannose were also found in this fraction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Leloir L. F. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc Natl Acad Sci U S A. 1970 May;66(1):153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming F. W. Dolichol phosphate, a coenzyme in the glycosylation of animal membrane-bound glycoproteins. Biochem Soc Trans. 1977;5(4):1223–1231. doi: 10.1042/bst0051223. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Bugge B., Jeanloz R. W. Glucosyltransferase activity in calf pancreas microsomes. Formation of dolichyl D[14C]glucosyl phosphate and 14C-labeled lipid-linked oligosaccharides from UDP-D-[14C]glucose. J Biol Chem. 1977 Apr 10;252(7):2271–2277. [PubMed] [Google Scholar]
- Herscovics A., Golovtchenko A. M., Warren C. D., Bugge B., Jeanloz R. W. Mannosyltransferase activity in calf pancreas microsomes. Formation of 14C-labeled lipid-linked oligosaccharides from GDP-D-[14C]mannose and pancreatic dolichyl beta-D-[14C]mannopyranosyl phosphate. J Biol Chem. 1977 Jan 10;252(1):224–234. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Martin-Barrientos J. Glycosylation of endogenous proteins through dolichol derivatives in reticulocyte plasma membranes. Biochim Biophys Acta. 1977 Nov 7;500(1):80–88. doi: 10.1016/0304-4165(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Parodi A. J. Synthesis of glycosyl-dolichol derivatives in bakers' yeast and their role in protein glycosylation. Eur J Biochem. 1977 May 2;75(1):171–180. doi: 10.1111/j.1432-1033.1977.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Krag S. S., Liu T. Effects of UDP-glucose addition on the synthesis of mannosyl lipid-linked oligosaccharides by cell-free fibroblast preparations. J Biol Chem. 1977 Mar 10;252(5):1780–1785. [PubMed] [Google Scholar]
- Scher M. G., Jochen A., Waechter C. J. Biosynthesis of glucosylated derivatives of dolichol: possible intermediates in the assembly of white matter glycoproteins. Biochemistry. 1977 Nov 15;16(23):5037–5044. doi: 10.1021/bi00642a015. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B. K., White D. A. The formation of lipid-linked sugars as intermediates in glycoprotein synthesis in rabbit mammary gland. Biochem J. 1978 Feb 15;170(2):273–283. doi: 10.1042/bj1700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. III. Comparison of oligosaccharide-lipids formed by slices from several tissues. J Biol Chem. 1976 Oct 25;251(20):6420–6425. [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White D. A., Pounder D. J., Hawthorne J. N. Phospholipase A 1 activity of guinea pig pancreas. Biochim Biophys Acta. 1971 Jul 21;242(1):99–107. doi: 10.1016/0005-2744(71)90091-x. [DOI] [PubMed] [Google Scholar]
- White D. A. The formation of lipid-linked sugars by cell-free preparations of lactating rabbit mammary gland. Biochem J. 1978 Mar 15;170(3):479–486. doi: 10.1042/bj1700479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Waechter C. J. A mannosyl-carrier lipid of bovine adrenal meddulla and rat parotid. Biochem J. 1975 Mar;146(3):645–651. doi: 10.1042/bj1460645. [DOI] [PMC free article] [PubMed] [Google Scholar]


