Abstract
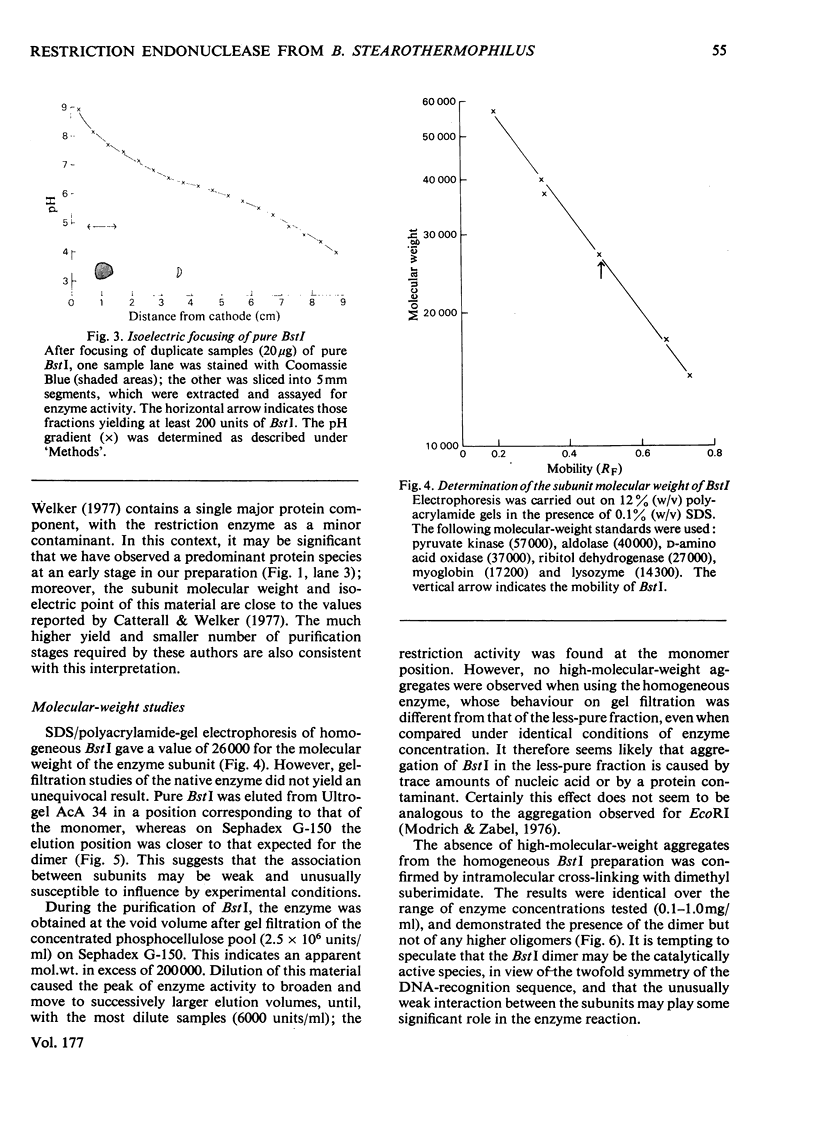
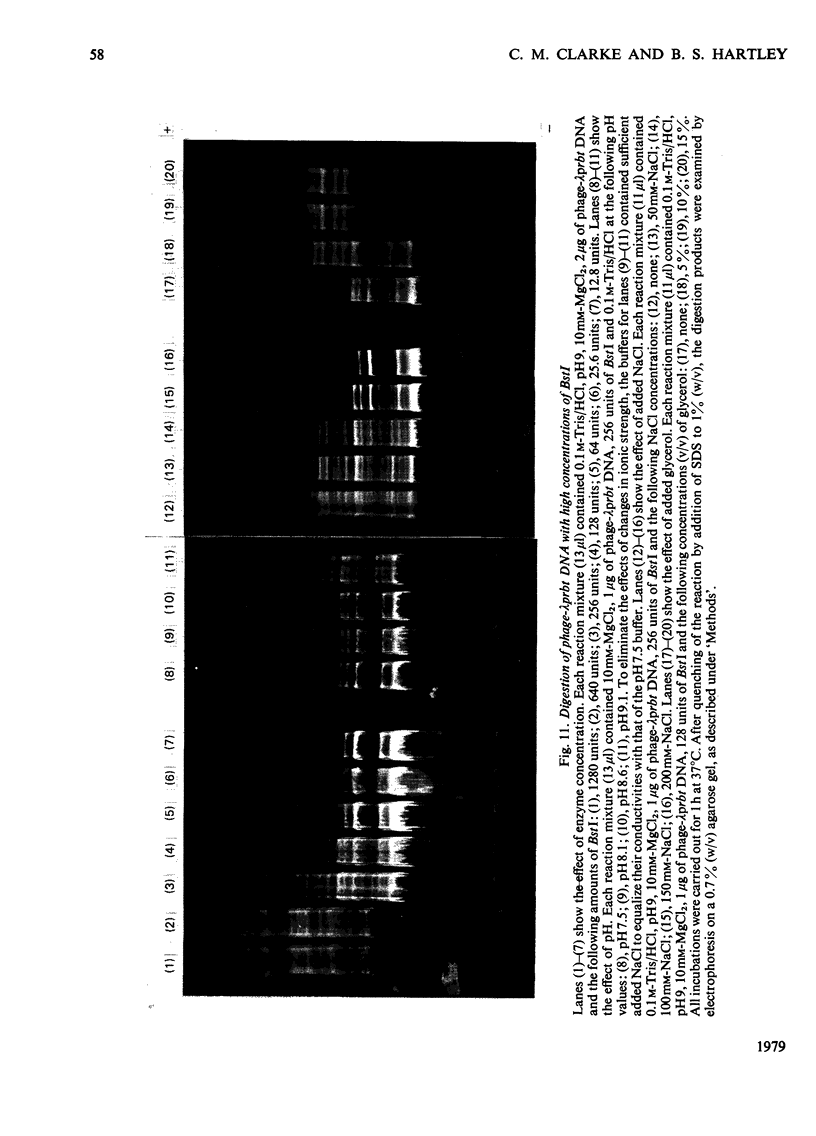
The restriction endonuclease BstI was purified from 70kg of Bacillus stearothermophilus. The final product is at least 97% pure as judged by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis; this major protein species co-migrates with the enzyme activity on native polyacrylamide-gel electrophoresis and isoelectric focusing. Pure restriction endonuclease BstI has a subunit mol.wt. of 26,000 and is probably a loosely associated dimer. The enzyme shows maximum activity at pH values between 7 and 9.5, and in the presence of 0.5-2mM-Mg2+. NaCl inhibits the restriction enzyme activity. Restriction endonuclease BstI cleaves DNA in a position identical with that cleaved by endonuclease BamHI (for Bacillus amyloliquefaciens), i.e.: (formula: see text). In the presence of high concentrations of enzyme, DNA cleavage occurs at secondary sites. This side-specificity is enhanced by the addition of glycerol. Preliminary studies indicate that these sites are of the type: (formula: see text).
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K., Trautner T. A. Restriction and modification in B. subtilis. Purification and general properties of a restriction endonuclease from strain R. Mol Gen Genet. 1975 Dec 30;143(1):13–23. doi: 10.1007/BF00269416. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Bruton C., Jakes R., Atkinson T. Gram-scale purification of methionyl-tRNA and tyrosyl-tRNA synthetases from Escherichia coli. Eur J Biochem. 1975 Nov 15;59(2):327–333. doi: 10.1111/j.1432-1033.1975.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Welker N. E. Isolation and properties of a thermostable restriction endonuclease (ENDO R-Bst1503). J Bacteriol. 1977 Feb;129(2):1110–1120. doi: 10.1128/jb.129.2.1110-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. Measurement of 32P activity in a liquid scintillation counter without the use of scintillator. Anal Biochem. 1968 Jan;22(1):70–73. doi: 10.1016/0003-2697(68)90260-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippes F. M. A new method for isolation of a restriction enzyme from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jun 4;58(3):586–596. doi: 10.1016/s0006-291x(74)80460-2. [DOI] [PubMed] [Google Scholar]
- Greene P. H., Poonian M. S., Nussbaum A. L., Tobias L., Garfin D. E., Boyer H. W., Goodman H. M. Restriction and modification of a self-complementary octanucleotide containing the EcoRI substrate. J Mol Biol. 1975 Dec 5;99(2):237–261. doi: 10.1016/s0022-2836(75)80143-4. [DOI] [PubMed] [Google Scholar]
- Haggerty D. M., Scheif R. F. Location in bacteriophage lamdba DNA of cleavage sites of the site-specific endonuclease from Bacillus amyloliquefaciens H. J Virol. 1976 May;18(2):659–663. doi: 10.1128/jvi.18.2.659-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heininger K., Hörz W., Zachau H. G. Specificity of cleavage by restriction nuclease from Bacillus subtilis. Gene. 1977 Jul;1(5-6):291–303. doi: 10.1016/0378-1119(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees N. D., Welker N. E. Restriction and modification of bacteriophage in Bacillus stearothermophilus. J Virol. 1973 Apr;11(4):606–609. doi: 10.1128/jvi.11.4.606-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Murray K. Nucleotide sequence analysis with polynucleotide kinase and nucleotide "mapping" methods. 5'-Terminal sequences of deoxyribonucleic acid from bacteriophages lambda and 424. Biochem J. 1973 Mar;131(3):569–582. doi: 10.1042/bj1310569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Gething M. J., Hartley B. S. Construction of intergeneric hybrids using bacteriophage P1CM: transfer of the Klebsiella aerogenes ribitol dehydrogenase gene to Escherichia coli. J Bacteriol. 1976 Feb;125(2):728–738. doi: 10.1128/jb.125.2.728-738.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Wilson G. A., Young F. E. Recognition sequence of specific endonuclease BamH.I from Bacillus amyloliquefaciens H. Nature. 1977 Jan 6;265(5589):82–84. doi: 10.1038/265082a0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Rigby P. W., Hartley B. S. Ribitol dehydrogenase from Klebsiella aerogenes. Purification and subunit structure. Biochem J. 1974 Sep;141(3):693–700. doi: 10.1042/bj1410693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Wu R., Bambara R., Jay E. Recent advances in DNA sequence analysis. CRC Crit Rev Biochem. 1975 Feb;2(4):455–512. doi: 10.3109/10409237509102550. [DOI] [PubMed] [Google Scholar]








