Abstract
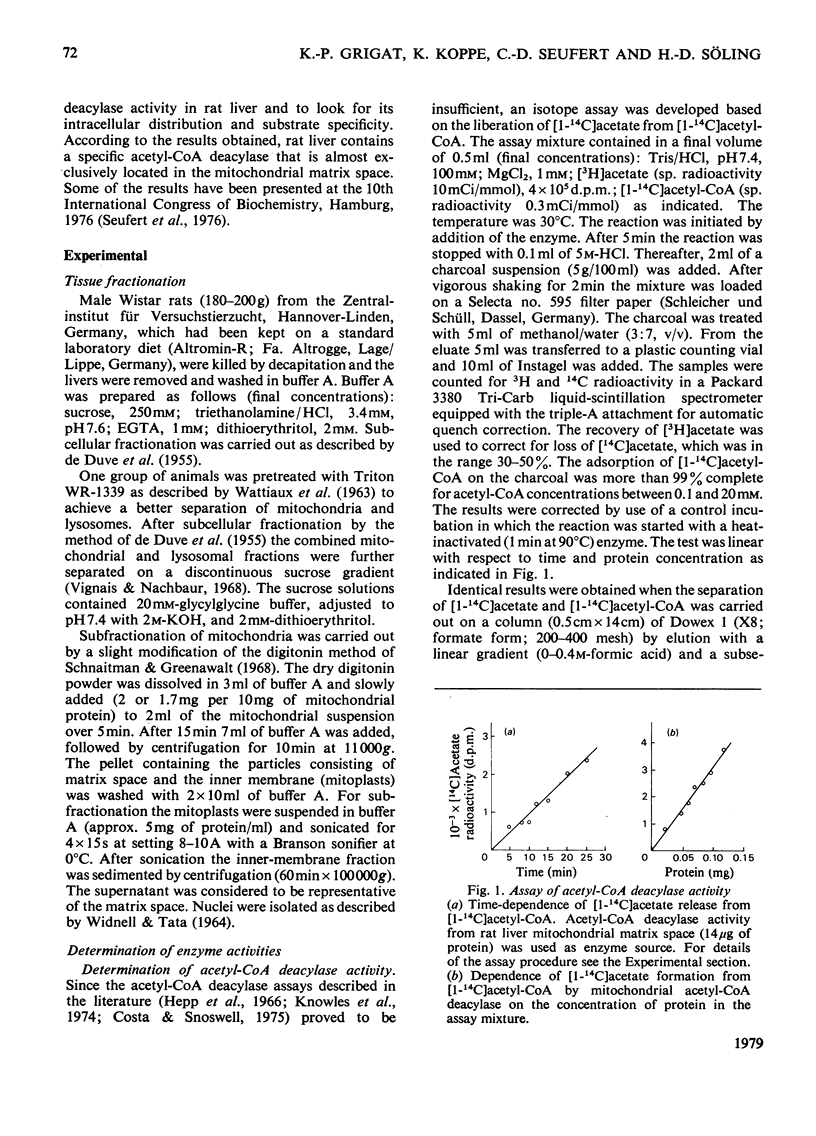
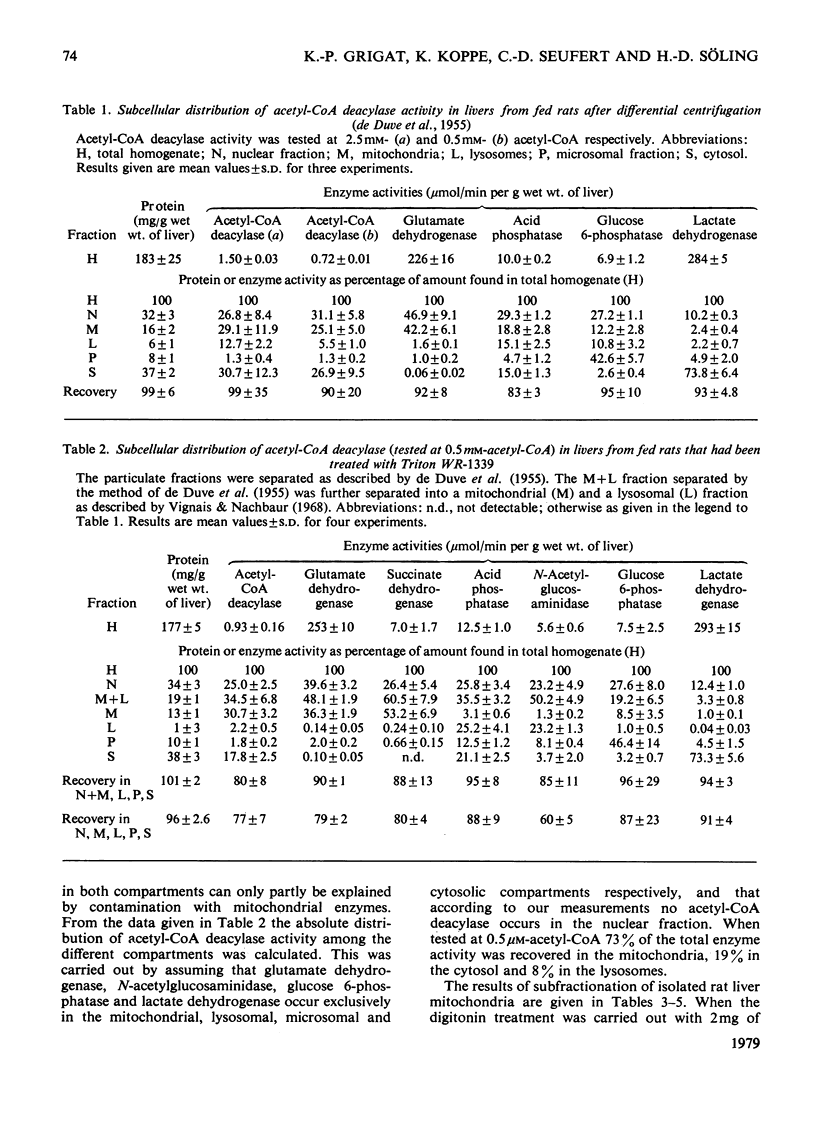
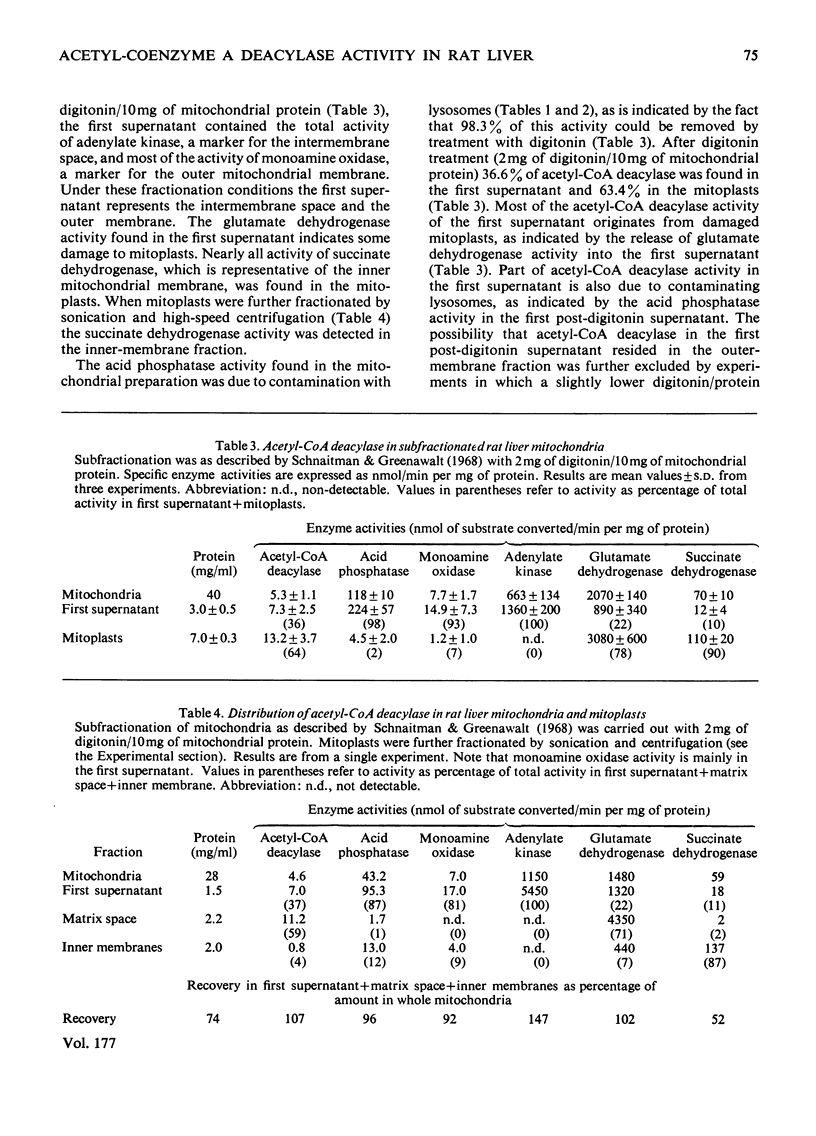
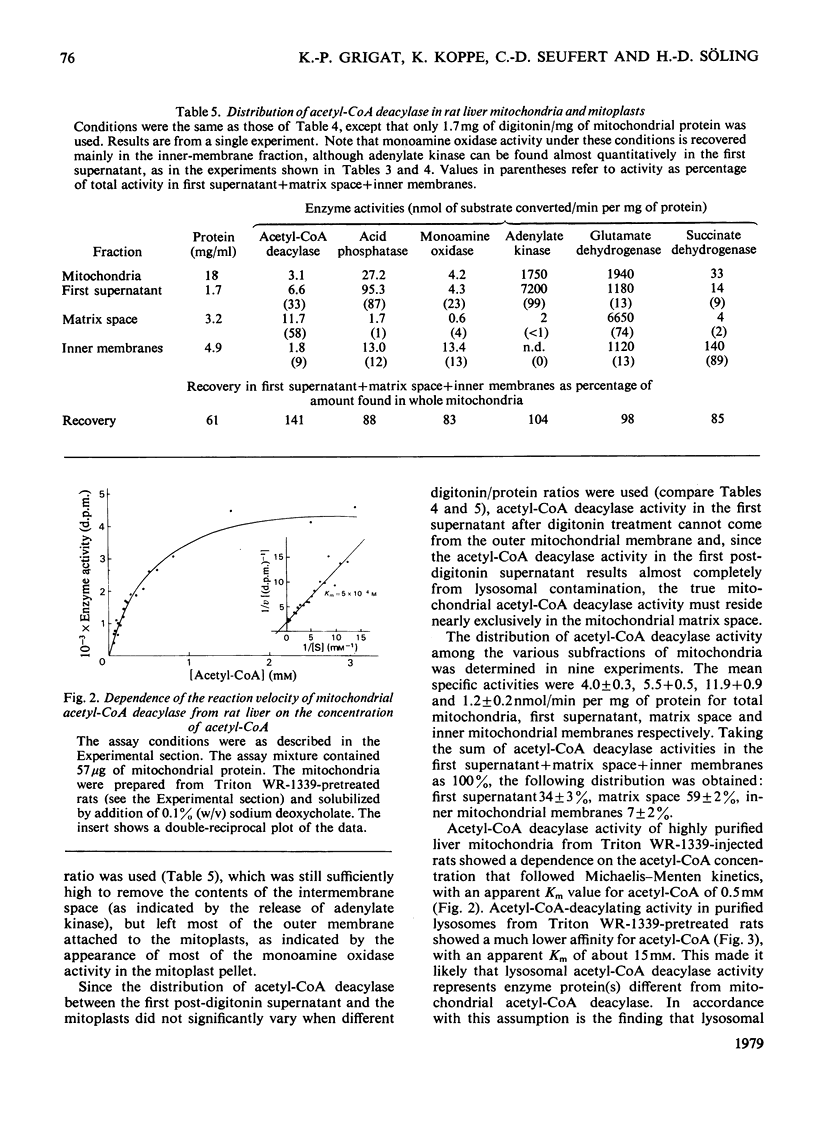
Whole liver and isolated liver mitochondria are able to release free acetate, especially under conditions of increased fatty acid oxidation. In the present paper it is shown that rat liver contains acetyl-CoA deacylase (EC 3.1.2.1) activity (0.72μmol/min per g wet wt. of liver at 30°C and 0.5mm-acetyl-CoA). At 0.5mm-acetyl-CoA 73% of total enzyme activity was found in the mitochondria, 8% in the lysosomal fraction and 19% in the postmicrosomal supernatant. Mitochondrial subfractionation shows that mitochondrial acetyl-CoA deacylase activity is restricted to the matrix space. Mitochondrial acetyl-CoA deacylase showed almost no activity with either butyryl- or hexanoyl-CoA. Acetyl-CoA hydrolase activity from purified rat liver lysosomes exhibited a very low affinity for acetyl-CoA (apparent Km>15mm compared with an apparent Km value of 0.5mm for the mitochondrial enzyme) and reacted at about the same rate with acetyl-, n-butyryl- and hexanoyl-CoA. We could not confirm the findings of Costa & Snoswell [(1975) Biochem. J. 152, 167–172] according to which mitochondrial acetyl-CoA deacylase was considered to be an artifact resulting from the combined actions of acetyl-CoA–l-carnitine acetyltransferase (EC 2.3.1.7) and acetylcarnitine hydrolase. The results are in line with the concept that free acetate released by the liver under physiological conditions stems from the intramitochondrial deacylation of acetyl-CoA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernson S. M. Acetyl-CoA hydrolase; activity, regulation and physiological significance of the enzyme in brown adipose tissue from hamster. Eur J Biochem. 1976 Aug 16;67(2):403–410. doi: 10.1111/j.1432-1033.1976.tb10705.x. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Costa N. D., McIntosh G. H., Snoswell A. M. Production of endogenous acetate by the liver in lactating ewes. Aust J Biol Sci. 1976 Mar;29(1-2):33–42. [PubMed] [Google Scholar]
- Costa N. D., Snoswell A. M. Acetyl-coenzyme A hydrolase, an artifact? The conversion of acetyl-coenzyme A into acetate by the combined action of carnitine acetyltransferase and acetylcarnitine hydrolase. Biochem J. 1975 Nov;152(2):167–172. doi: 10.1042/bj1520167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G. E., Weiss L. Transfer of C2-units across the mitochondrial membrane. Direct recording of citrate, acetate and acetoacetate production rates. Hoppe Seylers Z Physiol Chem. 1978 Jun;359(6):733–740. doi: 10.1515/bchm.1978.359.1.733. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Jarrett I. G., Filsell O. H., Ballard F. J. Production and utilization of acetate in mammals. Biochem J. 1974 Aug;142(2):401–411. doi: 10.1042/bj1420401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Robinson J. B., Jr, Mahan D. E., Koeppe R. E. Studies on rat brain acyl-coenzyme A hydrolase (short chain). Biochem Biophys Res Commun. 1976 Aug 23;71(4):959–965. doi: 10.1016/0006-291x(76)90748-8. [DOI] [PubMed] [Google Scholar]
- SRERE P. A., SEUBERT W., LYNEN F. Palmityl coenzyme A deacylase. Biochim Biophys Acta. 1959 Jun;33(2):313–319. doi: 10.1016/0006-3002(59)90118-0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert C. D., Graf M., Janson G., Kuhn A., Söling H. D. Formation of free acetate by isolated perfused livers from normal, starved and diabetic rats. Biochem Biophys Res Commun. 1974 Apr 8;57(3):901–909. doi: 10.1016/0006-291x(74)90631-7. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Subcellular distribution of key metabolites in isolated liver cells from fasted rats. FEBS Lett. 1976 Oct 15;69(1):265–271. doi: 10.1016/0014-5793(76)80701-6. [DOI] [PubMed] [Google Scholar]
- Snoswell A. M., Tubbs P. K. Deacylation of acetyl-coenzyme A and acetylcarnitine by liver preparations. Biochem J. 1978 May 1;171(2):299–303. doi: 10.1042/bj1710299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söling H. D., Kleineke J., Willms B., Janson G., Kuhn A. Relationship between intracellular distribution of phosphoenolpyruvate carboxykinase, regulation of gluconeogenesis, and energy cost of glucose formation. Eur J Biochem. 1973 Aug 17;37(2):233–243. doi: 10.1111/j.1432-1033.1973.tb02980.x. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Vignais P. M., Nachbaur J. A critical evaluation of the contamination, by lysosomes, of preparations of outer membrane of mitochondria. Biochem Biophys Res Commun. 1968 Oct 24;33(2):307–314. doi: 10.1016/0006-291x(68)90785-7. [DOI] [PubMed] [Google Scholar]
- Walter P., Anabitarte M. On the use of glutamate dehydrogenase as a mitochondrial marker enzyme for the determination of the intracellular distribution of rat liver pyruvate carboxylase. FEBS Lett. 1971 Jan 30;12(5):289–292. doi: 10.1016/0014-5793(71)80201-6. [DOI] [PubMed] [Google Scholar]
- Walter U., Söling H. D. Transfer of acetyl-units through the mitochondrial membrane: Evidence for a pathway different from the citrate pathway. FEBS Lett. 1976 Apr 1;63(2):260–266. doi: 10.1016/0014-5793(76)80107-x. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]


