Abstract
Docetaxel is a widely used first-line treatment for castration-resistant prostate cancer (CRPC). RhoB, a member of the Rho GTPase family, plays a major role in prostate cancer metastasis by modulating the PI3K-AKT signaling pathway. It is crucial in regulating cytoskeletal reassembly, cell migration, focal adhesion (FA) dynamics. To investigate RhoB’s function in prostate cancer, CRISPR/Cas9 gene editing technique was utilized to knock out the RhoB gene in prostate cancer cells. Successful gene editing was confirmed by using T7 endonuclease I (T7EI) assays and Sanger sequencing. Knocking out RhoB enhanced epithelial–mesenchymal transition (EMT) and decreased the IC50 value of docetaxel in RhoB-knockout PC-3 cells. This suggests increased sensitivity to docetaxel. Furthermore, RhoB knockout prompted the migration and invasion of prostate cancer cells, effects that were reversed upon RhoB overexpression. Interestingly, RhoB status did not significantly influence the cell cycle of prostate cancer cells. RNA sequencing of PC-3 cells with either overexpressed or knock-out RhoB revealed that RhoB regulates pathways involved in FA, ECM receptor interaction, and PI3K-AKT signaling. These pathways directly influence the EMT process, cell migration, and invasion in prostate cancer cells. Notably, RhoB overexpression activated PI3K-AKT signaling when PC-3 cells were treated with low concentration of DTXL (50 nM, 72 h). This activation reduced DTXL’s cytotoxicity, suggesting may confer chemoresistance via PI3K-AKT pathway activation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13762-4.
Keywords: Prostate cancer, RhoB, CRISPR/Cas9, RNA-Seq, Docetaxel
Introduction
Rho GTPases are pivotal regulators of cancer metastasis. These proteins influence actin cytoskeleton rearrangements and focal adhesion (FA) dynamics. Among the Rho subfamily isoforms- RhoA, RhoB, and RhoC-their sequences are highly similar, though their C-termini differ, where post-translational modifications frequently occur [1]. The RhoB protein, a member of this family, cycles between its active GTP-bound and inactive GDP-bound states. Unlike RAS, no constitutively active mutant forms of RhoB have been identified [2]. RhoB is localized to various cell compartments, including the cell membrane, endosomes, multivesicular bodies and nucleus. It regulates cytoskeleton reassembly, cell migration, and FA dynamics [3]. Its expression can be transiently induced by stimuli such as ultraviolet ray, cytokines, growth factors, and certain drugs. RhoB modulates the intracellular structure and function through pathways involving EGFR, Ras, and PI3K/Akt signaling [4, 5]. Feedback loops exist where EGFR, Ras, and PI3K-AKT signaling pathways downregulate RhoB, while RhoB regulates these pathways in turn [6, 7].
Although RhoB is generally downregulated in cancer and regarded as a tumor suppressor [8], its altered expression significantly impacts cancer progression. Unlike RhoA and RhoC, which are positively associated with metastasis, RhoB is negatively correlated with metastasis in some cancer types [9]. Rho GTPases, including RhoB, also play important regulatory roles in the epithelial–mesenchymal transition (EMT) process-a key driver of cancer metastasis [10]. EMT is involved in the disruption of epithelial cell-cell conjunctions, loss of cell polarity, and reorganization of the cytoskeleton. Several transcription factors, such as Snail, Zeb-1, and Slug, mediate EMT, enabling cancer cells to detach from the primary tumor, migrate through the basement membrane, invade the vasculature and metastasize [11]. In lung cancer, RhoB knockdown induces an elongated cell morphology, reduces E-cadherin expression, and increases Slug expression. RhoB interacts directly with PP2A to regulate AKT1 dephosphorylation [12]. RhoA and RhoC similarly regulate EMT in colon cancer, while Rac1 activation can induce EMT [13]. RhoB negatively regulates cell migration and invasion by modulating Akt activity and influencing EMT [14]. Interesting, high RhoB expression has been associated with worse overall survival in colorectal cancer patients, highlighting its complex role in cancer biology.
Studies have indicated that RhoB may regulate the cell cycle. In prostate cancer cells, RhoB knockout or overexpression has been linked to cell cycle changes. Specifically, RhoB interacts with cyclin B1 and CDK1 to induce G2/M phase arrest [15]. Among the different phases of cell cycle, RhoB levels peak during the S phase, whereas decline during the S/G2-M transition [16]. However, other studies have suggested that RhoB deletion dose not affect the cell cycle [17]. In addition to its role in the cell cycle, RhoB is implicated in autophagy regulation. Phosphorylated RhoB enhances its interaction with TSC2, and when the RhoB/TSC2 complex translocates to the lysosome, it effectively inhibits the mTORC1 functions, thereby activating autophagy [18]. RhoB also interacts with Beclin 1 and HSP90, thus enhancing the clearance of uropathogenic Escherichia coli predominately by preventing Beclin 1 degradation [19]. RhoB exhibits rapid turnover in cells, with its biosynthesis tightly regulated by various growth and stress stimuli. Unlike many other proteins, RhoB is degraded via the lysosomal pathway rather than the ubiquitin–proteasome system [20]. RhoB appears to act in a context-dependent manner. RhoB is essential for inducing apoptosis in transformed cells following DNA damage or treatment with Taxol [4].
In this study, CRISPR/Cas9 gene-editing technology was used to knock out the RhoB gene in PC-3 and DU145 cells. The effect of RhoB on EMT, DTXL sensitivity, cell migration and invasion, and the cell cycle of prostate cancer cells were examined. RNA sequencing (RNA-Seq) was performed to identify differentially expressed genes (DEGs) in PC-3 cells. RhoB was found to activate the PI3K-AKT signaling pathway in PC-3 cells treated with 50 nM DTXL for 72 h. Finally, a prostate cancer xenograft model was established to evaluate the effect of the RhoB gene on tumor growth.
Materials and methods
Materials
PC-3 cells and DU145 cells were purchased from the Chinese Academy of Typical Culture Collection Cell Bank (Shanghai, China). PC-3 and DU145 cells were cultured in DMEM/F12 and DMEM medium, respectively, which were supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. LentiCRISPR v2 was used for gene knockout, pCDH-CMV-MCS-EF1-copGFP-T2A-Puro was used for gene overexpression, and PCDH-CMV-mRFP-GFP-hLC3B-EF1A-Puro was used to analyze autophagy; all of these three vectors were obtained from Addgene company. The CRISPR/Cas9-mediated RhoB gene knockout was performed via sgRNA targeting at the exon of RhoB at approximately + 100 bp downstream of the ATG- initiation codon. Oligonucleotide sequences were cloned into lentiCRISPR v2. The insert sequences are listed in Table 1 and were synthesized by GENERAL BIOL company (Chuzhou, Anhui, China). NC sgRNA was used as a negative control. T7 endonuclease 1 (T7E1) was purchased from Vazyme (Nanjing, China), and the pUCm-T vector from Beyotime (Shanghai, China). The IPTG/X-Gal plate was used to screen the reconstructed amplicon, which was a white bacterial colony. The corresponding white colony was sequenced to assess the gene editing efficiency. Puromycin was purchased from MedChemExpress.
Table 1.
Insert oligonucleotide sequences used for CRISPR/Cas9 knockdown
| Primer | Forward | Reverse |
|---|---|---|
| NTC | CACCGCACCACGGTCCATACATACA | AAACTGTATGTATGGACCGTGGTGC |
| RhoB-1 | CACCGCACATAGTTCTCGAAGACGG | AAACCCGTCTTCGAGAACTATGTGC |
| RhoB-2 | CACCGCACCGTCTTCGAGAACTATG | AAACCATAGTTCTCGAAGACGGTGC |
Establishment of stable RhoB knockout or overexpressed cell lines
Three plasmid systems were used to pack the lentivirus system, and the virus was subsequently purified. The HEK293T cells were transfected with psPAX2, pMD2.G, and lentiCRISPR v2 or pCDH-CMV-MCS-EF1-copGFP-T2A-Puro plasmid to package the lentivirus. The PC-3 and DU145 cells were transfected with the lentivirus, and the successfully transfected cells were selected using puromycin (5 µg/mL). sgRNA1 generates the knockout cells and this cell line was used in subsequent studies.
T7EI assay
RhoB knock-out cancer cells were seeded into 6-well plate at a concentration of 5 × 105 cells/well, and their genomic DNA was isolated by using the Universal Genomic DNA Kit (CWBIO, CW2298) in accordance with the manufacturer’s instructions. The targeted genomic locus was amplified using PCR with the following primers: F: ATGGCGGCCATCCGCAAG, R: TCATAGCACCTTGCAGCA, and the amplicon sizes of the primers were 591 bp (Table 2). The amplicon was purified, and 200 ng of purified amplicons were denatured, reannealed, and digested with T7EI (Vazyme). To determine the DNA sequence of the targeted gene, the PCR product was TA-cloned into the pUCm-T vector.
Table 2.
Primer for RhoB full length amplication
| Primer | Forward | Reverse |
|---|---|---|
| RhoB full length | ATGGCGGCCATCCGCAAG | TCATAGCACCTTGCAGCA |
Western blotting
PC-3 or DU145 cells with RhoB gene knockout or overexpressed were seeded into 6-well culture plates. The cells were also treated with DTXL (50 nM) for 72 h. Total protein was extracted using a Westen blot lysis (Beyotime, China) and protein concentrations were determined using a BCA kit (Beyotime, China). Cell lysates were separated through 10–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% milk and incubated with primary antibodies overnight at 4 °C. The antibodies targeted the following proteins: β-catenin, vimentin, zonula occludens-1 (ZO-1), N-cadherin, E-cadherin, phospho-Akt (Thr308) (13038 S, CST), phospho-AKT (Ser473) (80455-1-RR, Proteintech), SRC (11097-1-AP, Proteintech), phospho-Src Family (Tyr416) (6943, CST), phospho-Src (Tyr30) (2105, CST), FAK (12636-1-AP, Proteintech), phospho-FAK (Tyr397) (8556, CST), mTOR( 66888-1-Ig, Proteintech), phospho-mTOR (Ser2448) (67778-1-Ig, Proteintech), NF-κB p65 (10745-1-AP, Proteintech), phospho-p44/42 MAPK (Erk1/2) (4370, CST), p44/42 MAPK (Erk1/2) (4695, CST), PI3 kinase p110 alpha (67071-1-Ig, Proteintech), PI3 kinase p110 beta (20584-1-AP, Proteintech), PI3 kinase p85 alpha (ab191606, Abcam), PI3 kinase p85 beta (ab180967, Abcam), and GAPDH. Secondary antibody incubation of the membrane followed for 1 h. Protein bands were visualized using a Tanon 2500R imaging system. Original gel files are in the Supplementary Information. Membrane was cut before hybridization with antibodies, but images of adequate length are absent.
RhoB knockout sensitizes the cancer cells to DTXL
PC-3 cells were plated in 96-well plates and treated with varying concentrations of DTXL for 24 h. Cell viability was assessed using the MTT assay. The IC50 value for DTXL was calculated based on cell inhibition rates. A calcein/PI cell viability/cytotoxicity assay kit was used to observe live and dead cells. To evaluate apoptosis, prostate cancer cells with different RhoB expression levels were treated with or without DTXL and analyzed using the Annexin V apoptosis assay.
The effect of RhoB on cell migration and invasion
The impact of RhoB on the migration and invasion of PC-3 or DU145 cells was evaluated using transwell chamber assays. For the migration assay, uncoated transwell chambers (8-µm pores, Corning, NY, USA) were used. For the invasion assay, 100 µL of Matrigel (356234; Corning Inc.), which was diluted by mixing with DMEM at a 1:8 ratio, was added to the upper chambers and incubated for 1 h at 37 °C. A total of 5 × 103 cells were seeded into the upper chambers, while the lower chambers contained 500 µL medium supplemented with 10% FBS. After incubation, cells on the upper surface of the transwell chambers were removed using cotton swabs, and those on the lower surface were fixed and stained with crystal violet for visualization.
Cell cycle analysis
Prostate cancer cells with RhoB knockout or overexpression were analyzed for cell cycle effects without synchronization. The cells were seeded into 6-well plates at a density of 1 × 105 cells/well. After 24 h, the cells were washed with cold PBS, fixed in 70% ethanol overnight at 4 °C, treated with RNase A for 30 min, and stained with propidium iodide (PI) for 30 min at 37 °C according to the manufacturer’s protocol. The cell cycle was measured using flow cytometry (BD FACS Caton, USA), and data were analyzed using FlowJo software (version 10.9).
RNA-Seq of RhoB knockout and overexpression in PC-3 cells
RNA-Seq was conducted to identify DEGs in PC-3 cells with RhoB knockout (KO group), RhoB overexpressed (OE group), and normal expression (CON group). Total RNA was extracted from the cells, and gene expression levels calculated as the mean value of log2(TPM + 1). Sequencing was performed using the BGISEQ, with paired-end reads of 150 bp (PE150). After the raw reads were filtered to obtain clean reads, the HISAT software was used to BLAST the clean reads and align them to the reference genome (GCF_000001405.39_GRCh38.p13). Differential gene expression was identified using a threshold of Q value ≤ 0.05 and │log2FC│ ≥ 1. Comparisons were made among KO vs. CON, OE vs. CON, and OE vs. KO groups. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of DEGs were performed using the Dr. Tom online system (BGI). Heatmaps were generated using the same online platform.
Immunofluorescence staining
Wildtype PC-3 cells and RhoB KO and RhoB OE PC-3 cells were treated with DTXL at a concentration of 50 nM for 72 h. The cells were incubated with phospho-FAK (Tyr397) (8556, CST) primary antibody overnight, followed by incubation with goat anti-rabbit IgG H&L Alexa Fluor® 488 at a 1:200 dilution for 2 h at room temperature. F-actin fibers were stained with actin-tracker Red-Rhodamine (Beyotime, Shanghai) at a 1:100 dilution for 30 min at room temperature, and nuclei were stained with DAPI. The distribution of p-FAK and F-actin fiber was observed using a fluorescence microscope (BX53, Olympus).
Tumor xenograft
To assess the effect of RhoB on tumor growth, subcutaneous xenografts were established in NOS-SCID mice (gender: male; age: 4–6 weeks; body weight: 14–21 g). Wildtype PC-3 cells, RhoB KO, and RhoB OE cells were prepared at a concentration of 5 × 106/mL, and 200 µL of each cell suspension was injected subcutaneously into the mice. To monitor tumor growth, 5 days later, the long and short diameters of the tumor were measured. These measurements were performed each day for 14 days post-tumor cell injection. Tumor volume was calculated using the formula: tumor volume (mm3) = 0.5 × L × W2, where L is the longest dimension and W is the perpendicular dimension. The mice were euthanized through cervical dislocation, and tumors were excised for histological and molecular analyses. Hematoxylin and eosin (H&E) staining was performed to evaluate histological changes. TUNEL staining was conducted to assess tumor cell apoptosis. Immunohistochemical (IHC) staining was used to detect the expression of RhoB (14326-1-AP, Proteintech), cleaved caspase-3 (GB11532-100), Ki67 (GB121141-100) on the tumor cells. All mice were maintained in a specific pathogen-free environment and sourced from Shanghai Model Organisms (Shanghai, China). The animal experimental procedures adhered to institutional ethical guidelines and were approved by the Ethics Committee of Jining No.1 People’s Hospital (License No. JNRM-2022-DW-062).
Immunohistochemistry and tunnel staining
Xenograft tumor tissues from mice were embedded in paraffin and sectioned. The sections were deparaffinized and rehydrated. To block endogenous peroxidase activity, slides were treated with 3% hydrogen peroxide for 25 min. Non-specific binding was minimized by blocking the untargeted protein with 3% BSA for 1 h at room temperature. Subsequently, the slides were incubated overnight at 4 °C with primary antibodies against RhoB, cleaved caspase3, Ki-67. After washing out the primary antibodies, the slides were incubated with secondary antibodies and stained using a DAB detection kit according to manufacturer’s protocols. Protein expression was scored and quantified using a staining index, calculated by multiplying the average staining intensity (0–3) by the extent of staining (0–4), resulting in a score ranging from 0 to 12. Two certified clinical pathologists independently analyzed and confirmed the results.
For TUNNEL staining, the sections were treated with protease K and then incubated with TDT enzyme, dUTP and buffer according to the manufactures’ instructions. The slides were counterstained with DAPI solution for 10 min at room temperature in the dark and observed under a fluorescent microscope.
Statistical analysis
Quantitative data from western blot signal analyses, DTXL IC50 determination, apoptosis rates, migration and invasion assays, cell cycle assessments, tumor growth curves, and IHC quantifications were analyzed for statistical significance using one-way ANOVA, followed by the Student–Newman–Keuls post hoc test (GraphPad Prism 9.5 software). Results are presented as mean ± SD, with statistical significance set at p < 0.05 unless otherwise indicated. The number of independent experiments, samples, or events is detailed in the respective figure legends.
Results
CRISPR/Cas9-mediated RhoB knockout in prostate cancer cells
PC-3 cells were transduced with sgRNA1 or sgRNA2 using lentiCRISPR v2 lentivirus. Cleavage of the target genome sequence was confirmed by the T7E1 assay, indicating effective targeting by sgRNA1 and sgRNA2, but not by the non-target control (NTC) sgRNA (Fig. 1A). Western blot analysis further confirmed that sgRNA1 and sgRNA2 induced complete RhoB knockout, while NTC did not affect RhoB expression. Overexpression lentivirus significantly increased RhoB protein levels (Fig. 1B). On the contrary, NTC treatment did not affect the alternation of RhoB protein expression, and overexpression lentivirus increased this expression remarkably (Fig. 1B). To confirm RhoB gene mutation, sgRNA1-, sgRNA2-, and NTC-treated PC-3 cells were used PCR amplifying the target sequence, subcloned into a T vector, and sequenced. Subsequently, we performed Sanger sequencing of the target regions in the cells with mutations, including insertions, deletions, and point mutations, resulting from non-homologous end-joining repair (Fig. 1C, D, E). Single clones of RhoB knockout PC-3 cells were isolated through limiting dilution, expanded, and observed microscopically (Fig. 1F).
Fig. 1.
RhoB knockout with CRISPR/Cas9 and evaluation of the knockout efficiency in PC-3. (A) T7E1 assay was performed to determine the knockout efficiency of CRISPR/Cas9 in PC-3 cells; red arrows indicate the cleaved products mediated by the T7E1 enzyme. (B) The RhoB protein expression after gene editing with CRISPR/Cas9 in PC-3 cells, while OE indicated the cells overexpressed in RhoB. (C) Sanger sequencing of the target region of the genome after editing with sgRNA1 CRSISPR-Cas9 in PC-3 cells, green dash rectangle indicates NGG. (D) Sanger sequencing of the target region of the genome after editing with sgRNA2 CRSISPR-Cas9 in PC-3 cells, green dash rectangle indicates NGG. (E) Sanger sequencing of the target region of the genome after editing with NTC sgRNA CRSISPR-Cas9 in PC-3 cells, green dash rectangle indicates NGG. (F) PC-3 cells after RhoB knockout with CRISPR/Cas9, and the stably transient cells after treatment with puromycin
EMT of prostate cancer cells after RhoB knockout
In PC-3 and DU145 cells, RhoB knockout led to decreased expression of epithelial markers E-cadherin and ZO-1 and increased expression of the mesenchymal marker vimentin, indicating effective induction of EMT. Conversely, RhoB overexpression resulted in increased E-cadherin expression, whereas decreased vimentin expression, signifying the occurrence of mesenchymal-epithelial transition. N-cadherin expression remained largely unchanged under both conditions. Furthermore, RhoB knockout elevated β-catenin expression levels, suggesting activation of the β-catenin signaling pathway (Fig. 2, Figure S1).
Fig. 2.
EMT-related protein expression in PC-3 cells with RhoB KO or RhoB OE. β-catenin, Vimentin, ZO-1, N-cadherin, and E-cadherin protein expressions were evaluated by Western blotting (A), and signal densities of EMT-related proteins were normalized to those of GAPDH (B). Data were expressed as the mean ± SD (n = 3), *P < 0.05, when compared with indicated group, one way ANOVA
Influence of RhoB on cytotoxicity of DTXL towards PC-3 cell
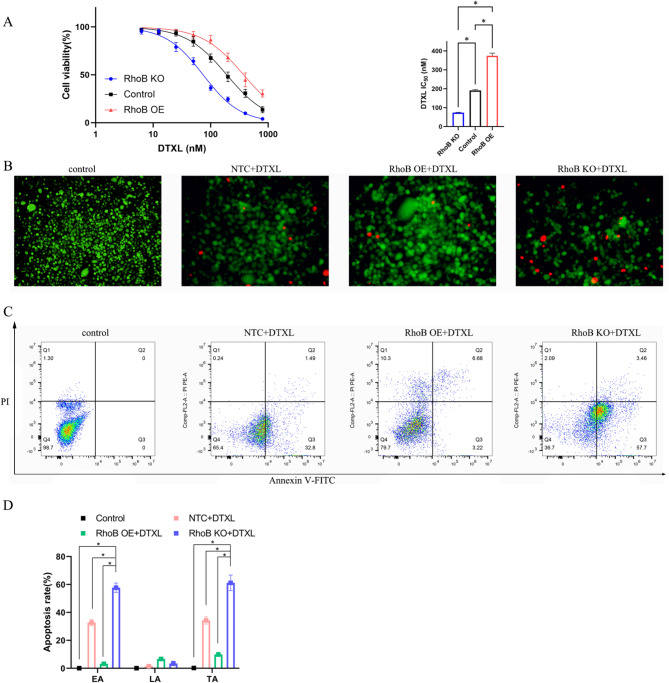
The IC50 values of DTXL in wildtype, RhoB KO, and RhoB OE PC-3 cells were 190.8, 74.06, and 374 nM, respectively. These results indicated that RhoB overexpression reduced DTXL sensitivity towards PC-3 cells, whereas RhoB knockout increased it (Fig. 3A).
Fig. 3.
In vitro anti-tumor ability of DTXL in RhoB knockout or overexpression in prostate cancer cells. (A) Cell viability and IC50 value of PC-3 cells toward DTXL treatment. (B) Calcein AM and PI double staining of PC-3 cells for live and dead cell visualization after RhoB knockout or overexpression in cancer cells treated with DTXL, green indicates live cells and red indicates dead cells. (C) PC-3 cell apoptosis was evaluated by flow cytometry after RhoB knockout or overexpressed cancer cells were treated with DTXL. (D) The Early apoptosis (EA) rate, late apoptosis (LA) rate, and total apoptosis (TA) rate of different groups were analyzed. Data were expressed as the mean ± SD, *P < 0.05, when compared with indicated group, one way ANOVA
Dead cell analysis further supported these findings. PC-3 cells treated with PBS but not with DTXL formed the control group, and dead cells were absent. Some dead cells were present in the control + DTXL group, signifying that DTXL had a cell-killing effect on the PC-3 cells. However, with RhoB gene knockout, the number of dead cells following DTXL treatment increased dramatically compared with that of the drug-treated wild-type prostate cancer cells. On the contrary, following RhoB gene knockout, the same concentration of DTXL enhanced cytotoxicity toward cancer cells compared to the control + DTXL group (Fig. 3B). Subsequently, PC-3 cell apoptosis after treatment with varying formulations was measured through flow cytometry. Total apoptosis rates (early plus late apoptosis rates) of the control, control + DTXL, RhoB OE + DTXL, and RhoB KO + DTXL groups were 0%, 34.29%, 9.90%, and 61.16%, respectively (Fig. 3C).
Migration and invasion assays
The results demonstrated that RhoB overexpression significantly inhibited prostate cancer cell migration compared to the control group. Both PC-3 and DU145 cells exhibited reduced migration (Fig. 4A). Conversely, RhoB knockout significantly enhanced cell migration, as evidenced by a substantial increase in the number of cells traversing the transwell membrane’s lower surface compared to the control group (Fig. 4A). Similarly, RhoB overexpression significantly suppressed the invasion of PC-3 and DU145 cells, the number of invading cells compared with the control group (Fig. 4B).
Fig. 4.
PC-3 cell and DU145 cell migration (A) and invasion (B) were analyzed by transwell methods after RhoB knockout or overexpression in cancer cells. Data were expressed as the mean ± SD (n = 3). *P < 0.05, when compared with indicated group, one way ANOVA
Cell-cycle analysis
The manipulation of RhoB expression-either through knockout or overexpression-did not lead to notable changes in the cell cycle distribution of PC-3 or DU145 cells. The proportions of cells in the G1, S, and G2/M phases were statistically comparable among the control, RhoB KO, and RhoB OE groups (Fig. 5A). Analysis from triplicate experiments confirmed the absence of significance across all experimental conditions (Fig. 5B).
Fig. 5.
Cell cycle analysis of prostate cancer cells treated with DTXL. RhoB was knockout or overexpressed in prostate cancer cells. (A) The G1 phase, S phase, and G2/M phase did not show any significant differences. (B) Data were expressed as the mean ± SD (n = 3). N.S no significant, when compared with indicated group, one way ANOVA
RNA-Seq results of RhoB gene manipulation in PC-3 prostate cancer cells
RNA sequencing was conducted to identify DEGs resulting from RhoB KO, OE, and CON in PC-3 cells. In the KO vs. CON comparison, 201 genes were upregulated, whereas 62 were downregulated. In the OE vs. CON comparison, 243 genes were upregulated, whereas 249 were downregulated. Finally, in the OE vs. KO comparison, 151 genes were upregulated, whereas 346 were downregulated (Fig. 6A, B).
Fig. 6.
RNA-seq analysis of RhoB knockout cells (KO), RhoB overexpressed cells (OE), and control cells (CON). (A) The number of differentially expressed genes (DEGs) among the KO, OE, and CON groups. (B) Venn diagram depicting the number of the DEGs in the KO/CON, OE/KO, and OE/CON groups. (C) GO cellular component enrichment analyses of DEGs among KO/CON, OE/CON, and OE/KO. (D) The KEGG pathway enrichment analysis of DEGs among KO/CON, OE/CON, and OE/KO
GO enrichment analysis identified significant terms enriched in the DEGs. For KO vs. CON, the enriched terms included FA, adherens junctions, cell–cell junctions, and collagen-containing extracellular matrix. In OE vs. CON, FA, adherens junction, lamellipodia, extracellular exosomes, and cell junctions were enriched. Similarly, for OE vs. KO, enriched terms included FA, adherens junctions, cell junctions, extracellular exosomes, and endoplasmic reticulum. These findings suggest that FA of PC-3 cells and related cellular structures are affected by RhoB gene manipulation (Fig. 6C).
The KEGG pathway analysis revealed enrichment in multiple signaling pathways. In KO vs. CON, the enriched pathways included PI3K-AKT, ECM–receptor interaction, FA, proteoglycans in cancer, cell adhesion molecules, and oxidative phosphorylation. For OE vs. CON, the enriched pathways included proteoglycans in cancer, FA, pathways in cancer, oxidative phosphorylation, and adherens junctions. In OE vs. KO, the enriched pathways were PI3K-AKT signaling, adherens junction, FA, proteoglycans in cancer, and pathways in cancer. These results indicated that the PI3K-AKT-signaling pathway is significantly influenced by RhoB KO or OE (Fig. 6D).
In the FA-signaling pathway of OE vs. CON, the DEGs included ITGA2, EGFR, VEGFC, PPP1CB, LAMC2, TNC, VEGFA, LAMA3, THBS1, and LAMB3 (Fig. 7A). In the PI3K-AKT-signaling pathway of OE vs. CON, the DEGs were THBS1, AREG, ITGA2, EGFR, VEGFC, JAK1, ITGA5, LAMB2, COL6A3, TGFA, ERBB3, IL4R, CDKN1A, and IL6 (Fig. 7B). In the ECM–receptor interaction-signaling pathway of KO vs. CON, the DEGs were NPNT, COL6A3, ITGAV, COL4A1, ITGA5, COL4A2, COL1A1, and DAG1 (Fig. 7C). Pathways in cancer signaling were enriched in the DEGs of KO vs. CON, and the DEGs included TGFB2, IL6, MSH2, COL4A2, NOTCH2, MSH6, IL4R, NCOA3, EGLN1, RUNX1, STAT2, NOTCH3, and AKT3 (Fig. 7D).
Fig. 7.
Heatmap of RNA-seq analysis of RhoB KO cells, RhoB OE cells, and the control cells. (A) The DEG is enriched from the focal adhesion pathway between the OE and CON groups. (B) The DEG enriched from the PI3K-AKT pathway between the OE and CON groups. (C) The DEG is enriched from the ECM receptor interaction pathway between the KO and CON groups. (D) The DEG enriched from the cancer pathway between the KO and CON groups
RhoB activates PI3K-AKT signaling at low DTXL concentration
Our study revealed that RhoB overexpression reduces the sensitivity of PC-3 cells to DTXL (Fig. 3). Low DTXL concentrations in prostate cancer tissues, often due to limited drug distribution, necessitate further investigation into the effects of RhoB on DTXL sensitivity. To this end, we selected a DTXL concentration of 50 nM, below the IC50 for PC-3 cells with RhoB KO or OE. At 50 nM DTXL, RhoB overexpression enhanced AKT phosphorylation at T308, with increased levels of p-T308-AKT observed in PC-3 cells treated for 72 h. However, phosphorylation at S473-AKT showed no remarkable change. Regarding Src signaling, p-Y530-Src levels remained unchanged in DTXL-treated cells, whereas p-Y416-Src decreased in RhoB-overexpressing cancer cells, indicating downregulation independent of DTXL treatment. Moreover, p-FAK levels increased after DTXL treatment, but no remarkable changes were noted in the expression of PI3K subunits (p110α, p110β, p85α, or p85β), regardless of RhoB expression or DTXL treatment. These findings suggest that RhoB OE activates the PI3K-AKT-signaling pathway, whereas inhibits Src activity in response to low-dose DTXL (50 nM, 72 h) treatment, but RhoB did not affect Src activity (Fig. 8A).
Fig. 8.
RhoB activates the PI3K-AKT signaling pathway upon DTXL treatment in prostate cancer cells. (A) In the presence of DTXL, RhoB OE can activate AKT (p-308) while, in the absence of DTXL, RhoB OE can inhibit AKT (p-308). (B) Immunofluorescence co-staining of p-FAK and F-actin in prostate cancer cells with RhoB different expression status after DTXL treatment. F-actin and p-FAK immunostaining. Representative microphotographs are shown, objective 20×, F-actin (red), p-FAK (green), nucleus staining (blue)
Immunofluorescence results corroborated these observations, showing increased p-FAK in the cytoplasm of cell groups treated with 50 nM DTXL for 72 h (Fig. 8A). Additionally, F-actin levels decreased in the wild-type and RhoB KO groups following DTXL treatment, but remained unaffected in the RhoB OE group. This resistance to F-actin reduction may contribute to decreased DTXL sensitivity in RhoB OE cells (Fig. 8B).
RhoB knockout decreases tumor growth in prostate cancer xenografts
To evaluate the in vivo effects of RhoB, prostate cancer xenografts were established. Tumor growth curves indicated that RhoB OE significantly inhibited tumor growth (Fig. 9A, B). While cancer cell morphology exhibited no discernible changes (Fig. 9E). TUNEL staining revealed increased apoptosis in RhoB OE cells compared with control or RhoB KO cells (Fig. 9F). IHC analysis further confirmed elevated cleaved caspase 3 levels and reduced Ki67 expression in the RhoB OE group, indicating decreased proliferation and increased apoptosis (Fig. 9G-J). Data from The Cancer Genome Atlas (TCGA) database unveiled that reduced RhoB expression in prostate adenocarcinoma (PRAD) tissues compared with normal prostate tissues (Fig. 9C). Moreover, patients with RhoB overexpression demonstrated significantly better prognoses than those with low/medium RhoB expression (Fig. 9D).
Fig. 9.
Prostate cancer xenograft to study the RhoB effects on cancer progression. (A, B) The growth curve displays that RhoB OE could inhibit cancer growth. (C, D) TCGA data showed the RhoB expression in normal prostate tissues and prostate adenocarcinoma (PRAD) tissues, while the survival curve demonstrated that the RhoB expression affected the survival time of prostate cancer patients. (E) H&E staining of tumor tissues dissected from the prostate cancer xenograft. (F) TUNEL staining of tumor tissues with RhoB overexpression or knockout. (G, H, I) IHC staining of Ki67, cleaved caspase 3 and RhoB proteins in the tumor tissues with RhoB overexpression or knockout, objective 20×. (J) Quantification of IHC staining for Ki67, cleaved caspase 3, and RhoB proteins in prostate cancer xenograft. *P < 0.05, when compared with indicated group, one way ANOVA
Discussion
EMT is a critical process driving prostate cancer metastasis and is regulated by various molecular mechanisms [21]. The incidence of prostate cancer has risen in China, especially in developed regions, likely due to increased PSA screening and improved biopsy techniques [22]. RhoB has previously been implicated in Taxol-induced apoptosis of transformed cells, where it likely plays a pro-apoptotic role [17]. Some studies have shown that EMT is closely related to cancer cell migration and invasion, and is crucial for cancer metastasis [23, 24]. In this study, the RhoB gene in PC-3 and DU145 cells was successfully knocked out using the CRISPR/Cas9 gene editing method, which was verified at the gene and protein level. sgRNA targeted the exon of this gene at approximately + 100 bp downstream of the ATG initiation codon, thereby effectively inducing INDEL in the RhoB gene, as confirmed through Sanger sequencing. No cell type-specific off-target effects were noted; these effects depend on the DSB repair pathway of the specific cell type [25].
Our study demonstrated that RhoB knockout induces EMT in prostate cancer cells, as evidenced by decreased E-cadherin and ZO-1 levels and increased vimentin and β-catenin expression [26]. However, N-cadherin levels remained unchanged in our study. Conversely, RhoB OE inhibited EMT-associated proteins expression, reducing the migration and invasion capabilities of prostate cancer cells. These findings align with previous studies on lung cancer cells, where RhoB KO augmented migration and invasion. RhoB changes the morphology of bronchial cells, RhoB KO induces elongation of the cells, and RhoB OE induces the round-shaped cells [12, 26, 27]. Thus, RhoB knockout can induce the mesenchymal phenotype of cancer cells and increase motility. RNA-Seq analysis revealed that these genes pertaining to focal adhesion, adherens junction, and cell–cell junction were enriched in the DEGs of KO vs. CON. This suggests that RhoB regulates genes involved in the aforementioned processes, thereby playing a crucial role in maintaining cell polarity and adhesion. Previous study has described RhoB as an oncogene in breast cancer that phosphorylates the AKT protein and upregulates the expression of hypoxia-inducible factor-1α (HIF1α) under hypoxic conditions. However, our findings indicate that its role is context-dependent and influenced by the tumor microenvironment [4, 28].
DTXL, a widely used chemotherapeutic agent for prostate cancer, often encounters resistance with prolonged therapy [29]. In our study, RhoB KO enhanced DTXL cytotoxicity, as reflected by reduced IC50 values and increased apoptosis in PC-3 cells. The Calcein AM/PI assay exhibited that the number of dead cells was high in the RhoB KO groups. Hence, RhoB KO appears to sensitize prostate cancer cells to DTXL, thereby resulting in their apoptosis. Conversely, RhoB OE conferred resistance to DTXL in PC-3 cells, likely through the activation of the PI3K-AKT signaling pathway. Specifically, RhoB OE increased p-T308-AKT levels under low-dose DTXL (50 nM, 72 h) but did not affect p-S473-AKT. Furthermore, RhoB OE reduced p-Y416-Src levels while leaving p-Y530-Src unchanged. Low-dose DTXL increased p-FAK levels, and RhoB KO reduced p85α levels. Immunofluorescence confirmed that RhoB OE increased p-FAK levels and stabilized F-actin levels under DTXL treatment (50 nM, 72 h). In cancer cells not treated with DTXL, RhoB loss seems to increase F-actin levels, which was consistent with Transwell and EMT results. RhoB exerts different effects on AKT in cancer cells treated with DTXL and those untreated. DTXL may reverse the effect of RhoB on AKT. In a study, RhoB OE endowed lung cancer cells with resistance to erlotinib by activating AKT, and the RhoB/AKT axis played a crucial role in the induction of this resistance [30]. Another study reported that RhoB OE was related to the worse survival of colorectal cancer patients who received chemotherapy [31]. RhoB OE may induce resistance to chemotherapeutic agents. Our results also indicated that RhoB OE induces resistance to DTXL in PC-3 cells. In one study, RhoB degradation increased FA formation, thereby increasing cell invasion, and when RhoB interacted with ARF6, RhoB KO enhanced F-actin activity; this is consistent with our results.
In conclusion, RhoB plays a multifaceted role in prostate cancer by regulating EMT, DTXL sensitivity, and cell migration and invasion. RhoB KO, achieved through CRISPR/Cas9, disrupted cellular junctions and basal–apical polarity, enhancing motility and drug sensitivity. Conversely, RhoB OE activated the PI3K-AKT-signaling pathway, inhibited Src activity, and conferred resistance to low-dose DTXL. These results were verified using RNA-Seq, which also indicated that RhoB is involved in the cell FA and adherens junctions. However, the gene did not affect the cell cycle. Furthermore, in PC-3 cells that were treated with low-dose DTXL (50 nM, 72 h), RhoB OE activated p-T308-AKT and inhibited p-Y416-Src. However, the accurate mechanism needs to be elucidated in the future. In vivo studies further demonstrated that RhoB OE reduced tumor growth and increased apoptosis, suggesting a potential therapeutic role for targeting RhoB in combination with chemotherapeutic agents. Our findings highlight the possibility of enhancing DTXL efficacy by combining it with AKT inhibitors in RhoB-overexpressing prostate cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
NA.
Author contributions
Tiantian Sheng and Hang Su did most of the experiment, and Lu Yao collected the data, Zhen Qu analyzed the data, Hui Liu prepared the Figures, Xiangyu Zhang and Wenjuan Shao wrote the main manuscript text. All authors have read and approved the manuscript.
Funding
This work was financially supported through grants from the Natural Science Foundation of Shandong Province (Nos. ZR2023MH260 and ZR2017QH005), the National Natural Science Foundation of China (Grant No. 81803097), Doctoral Fund of Jining No.1 People’s Hospital (2022-BS-002) and Key Research and Development Plan of Jining (2022YXNS113).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics statement
All experiments including animal experiments were approved by the Ethics Committee of Jining No.1 People’s Hospital approved this study (License No. JNRM-2022-DW-062). No human experiments were conducted in this study.
Informed consent
N/A.
Registry and the registration no. of the study/trial
N/A.
Animal studies
Yes.
Consent for publication
All authors have consented to publication of this manuscript. All authors have read and approved the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiantian Sheng and Hang Su contributed equally to this work.
Contributor Information
Wenjuan Shao, Email: Shaowenjuan1022@163.com.
Xiangyu Zhang, Email: zhangxiangyu666@126.com.
References
- 1.Jansen S, Gosens R, Wieland T, Schmidt M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol Ther. 2018;183:1–21. [DOI] [PubMed] [Google Scholar]
- 2.Hodge RG, Schaefer A, Howard SV, Der CJ. RAS and RHO family GTPase mutations in cancer: twin sons of different mothers? Crit Rev Biochem Mol Biol. 2020;55(4):386–407. [DOI] [PubMed] [Google Scholar]
- 3.Zaoui K, Smith HW, Park M, Duhamel S. ARF6 controls RHOB targeting to endosomes regulating cancer cell invasion. Mol Cell Oncol. 2020;7(5):1766932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendergast GC. Actin’ up: RhoB in cancer and apoptosis. Nat Rev Cancer. 2001;1(2):162–8. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez E, Cahatol I, Bailey CAR, Lafargue A, Zhang N, Song Y, Tian H, Zhang Y, Chan R, Gu K, Zhang ACC, Tang J, Liu C, Connis N, Dennis P, Zhang C. Regulation of RhoB gene expression during tumorigenesis and aging process and its potential applications in these processes. Cancers (Basel). 2019;11(6):818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang K, Delarue FL, Sebti SM. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene. 2004;23(5):1136–45. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Huang W, Wang X, Zhang J, Tao T, Zheng Y, Liu S, Yang J, Chen ZS, Cai CY, Li J, Wang H, Fan Y. Hsa-miR-3178/RhoB/PI3K/Akt, a novel signaling pathway regulates ABC transporters to reverse gemcitabine resistance in pancreatic cancer. Mol Cancer. 2022;21(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju JA, Gilkes DM. RhoB: team oncogene or team tumor suppressor?? Genes (Basel). 2018, 9(2):67. [DOI] [PMC free article] [PubMed]
- 9.Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc. 2013;251(3):242–9. [DOI] [PubMed] [Google Scholar]
- 10.Crosas-Molist E, Samain R, Kohlhammer L, Orgaz JL, George SL, Maiques O, Barcelo J, Sanz-Moreno V. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102(1):455–510. [DOI] [PubMed] [Google Scholar]
- 11.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18(2):128–34. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet E, Calvayrac O, Mazières J, Lajoie-Mazenc I, Boubekeur N, Favre G, Pradines A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A Inhibition. Oncogene. 2016;35(14):1760–9. [DOI] [PubMed] [Google Scholar]
- 13.Zacharopoulou N, Tsapara A, Kallergi G, Schmid E, Alkahtani S, Alarifi S, Tsichlis PN, Kampranis SC, Stournaras C. The epigenetic factor KOM2B regulates EMT and small GTPases in Colon tumor cells. Cell Physiol Biochem. 2018;47(1):368–77. [DOI] [PubMed] [Google Scholar]
- 14.Calvayrac O, Pradines A, Favre G. RHOB expression controls the activity of serine/threonine protein phosphatase PP2A to modulate mesenchymal phenotype and invasion in non-small cell lung cancers. Small GTPases. 2018;9(4):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Niu S, Ma X, Zhang P, Gao Y, Fan Y, Pang H, Gong H, Shen D, Gu L, Zhang Y, Zhang X. RhoB acts as a tumor suppressor that inhibits malignancy of clear cell renal cell carcinoma. PLoS ONE. 2016;11(7):e0157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalcman G, Closson V, Linarès-Cruz G, Lerebours F, Honoré N, Tavitian A, Olofsson B. Regulation of Ras-related RhoB protein expression during the cell cycle. Oncogene. 1995;10(10):1935–45. [PubMed] [Google Scholar]
- 17.Liu AX, Cerniglia GJ, Bernhard EJ, Prendergast GC. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc Natl Acad Sci U S A. 2001;98(11):6192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Zeng T, Zhang X, Liu C, Wu Z, Yao L, Xie C, Xia H, Lin Q, Xie L, Zhou D, Deng X, Chan HL, Zhao TJ, Wang HR. ATR/Chk1 signaling induces autophagy through sumoylated RhoB-mediated lysosomal translocation of TSC2 after DNA damage. Nat Commun. 2018;9(1):4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao C, Yu M, Pei G, Ma Z, Zhang L, Yang J, Lv J, Zhang ZS, Keller ET, Yao Z, Wang Q. An infection-induced RhoB-Beclin 1-Hsp90 complex enhances clearance of uropathogenic Escherichia coli. Nat Commun. 2021;12(1):2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Sala D, Boya P, Ramos I, Herrera M, Stamatakis K. The C-terminal sequence of RhoB directs protein degradation through an endo-lysosomal pathway. PLoS ONE. 2009;4(12):e8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel RL, Miller KO, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 22.Ju W, Zheng R, Zhang S, Zeng H, Sun K, Wang S, Chen R, Li L, Wei W, He J. Cancer statistics in Chinese older people, 2022: current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea. Sci China Life Sci. 2023;66(5):1079–91. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu C, Li X, Wu D, Xia S, Chen J, Qian WP, Yang L, Osunkoya AO, Boise L, Vertino PM, Zhao Y, Li M, Chen HR, Kowalski J, Kucuk O, Zhou W, Dong JT. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat Commun. 2021;12(1):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Li P, Huang H, Ye X, Chen W, Xu G, Zhang F. Androgen receptor regulates eIF5A2 expression and promotes prostate cancer metastasis via EMT. Cell Death Discov. 2021;7(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan J, Lu G, Xie Z, Lou M, Luo J, Guo L, Zhang Y. Genome-wide identification of CRISPR/Cas9 off-targets in human genome. Cell Res. 2014;24(8):1009–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousquet E, Mazières J, Privat M, Rizzati V, Casanova A, Ledoux A, Mery E, Couderc B, Favre G, Pradines A. Loss of RhoB expression promotes migration and invasion of human bronchial cells via activation of AKT1. Cancer Res. 2009;69(15):6092–9. [DOI] [PubMed] [Google Scholar]
- 27.Sun M, Nie FQ, Zang C, Wang Y, Hou J, Wei C, Li W, He X, Lu KH. The pseudogene DUXAP8 promotes Non-small-cell lung Cancer cell proliferation and invasion by epigenetically Silencing EGR1 and RHOB. Mol Ther. 2017;25(3):739–51. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Ju JA, Godet I, DiGiacomo JW. GilkesDM. RhoB is regulated by hypoxia and modulates metastasis inbreast cancer. Cancer Rep. 2020;3:e1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashemi M, Zandieh MA, Talebi Y, Rahmanian P, Shafiee SS, Nejad MM, Babaei R, Sadi FH, Rajabi R, Abkenar ZO, Rezaei S, Ren J, Nabavi N, Khorrami R, Rashidi M, Hushmandi K, Entezari M, Taheriazam A. Paclitaxel and docetaxel resistance in prostate cancer: molecular mechanisms and possible therapeutic strategies. Biomed Pharmacother. 2023;160:114392. [DOI] [PubMed] [Google Scholar]
- 30.Calvayrac O, Mazières J, Figarol S, Marty-Detraves C, Raymond-Letron I, Bousquet E, Farella M, Clermont-Taranchon E, Milia J, Rouquette I, Guibert N, Lusque A, Cadranel J, Mathiot N, Savina A, Pradines A, Favre G. The RAS-related GTPase RHOB confers resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer via an AKT-dependent mechanism. EMBO Mol Med. 2017;9(2):238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopsida M, Liu N, Kotti A, Wang J, Jensen L, Jothimani G, Hildesjo C, Haapaniemi S, Zhong W, Pathak S, Sun XF. RhoB expression associated with chemotherapy response and prognosis in colorectal cancer. Cancer Cell Int. 2024;24(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.