Abstract
The pyruvate dehydrogenase multienzyme complex was isolated from Escherichia coli grown in the presence of [35S]sulphate. The three component enzymes were separated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and the molar ratios of the three polypeptide chains were determined by measurement of the radioactivity in each band. The chain ratio of lipoamide dehydrogenase to lipoate acetyltransferase approached unity, but there was a molar excess of chains of the pyruvate decarboxylase component. The 35S-labelled complex was also used in a new determination of the total lipoic acid content. It was found that each polypeptide chain of the lipoate acetyltransferase component appears to bear at least three lipoyl groups.
Full text
PDF


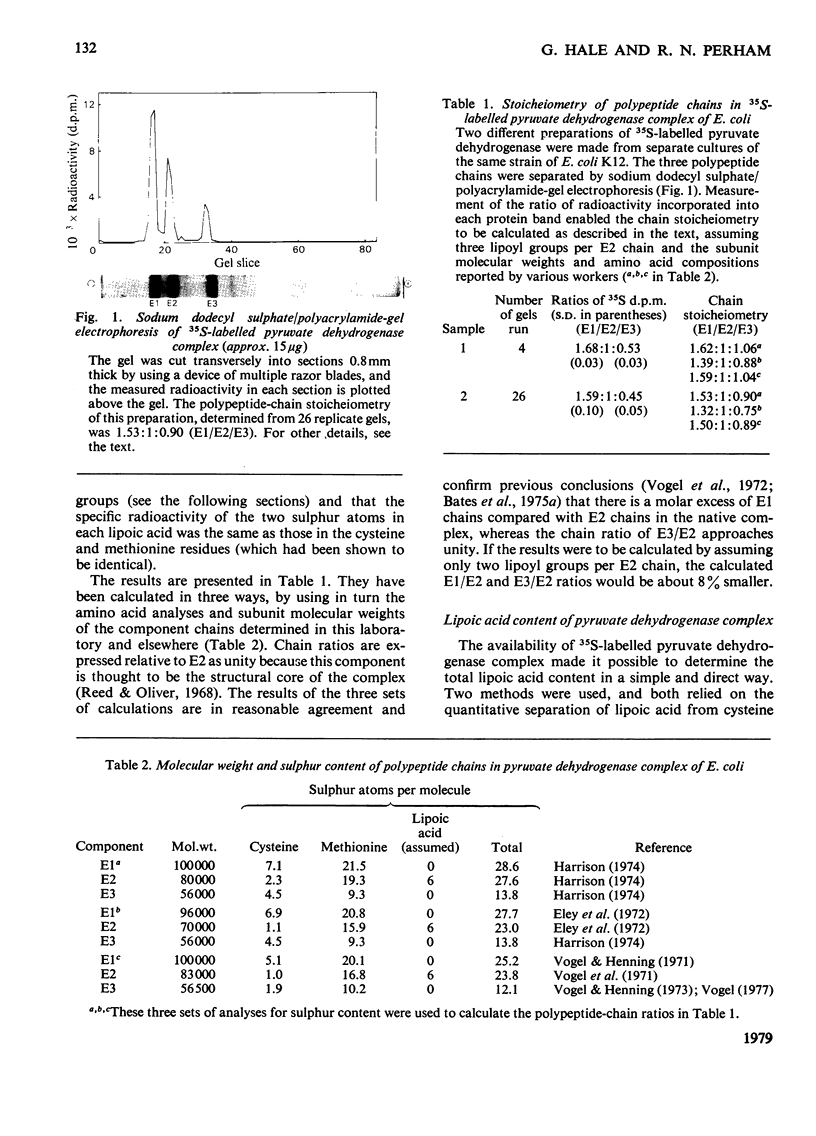
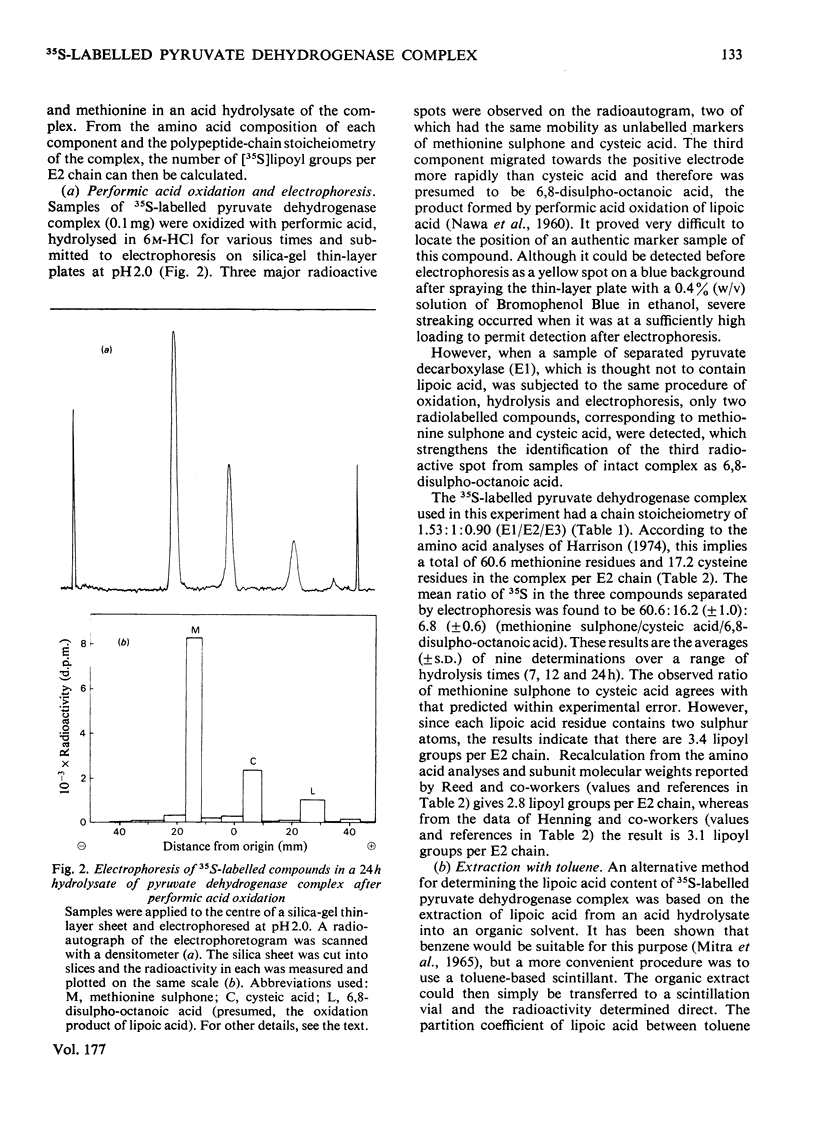
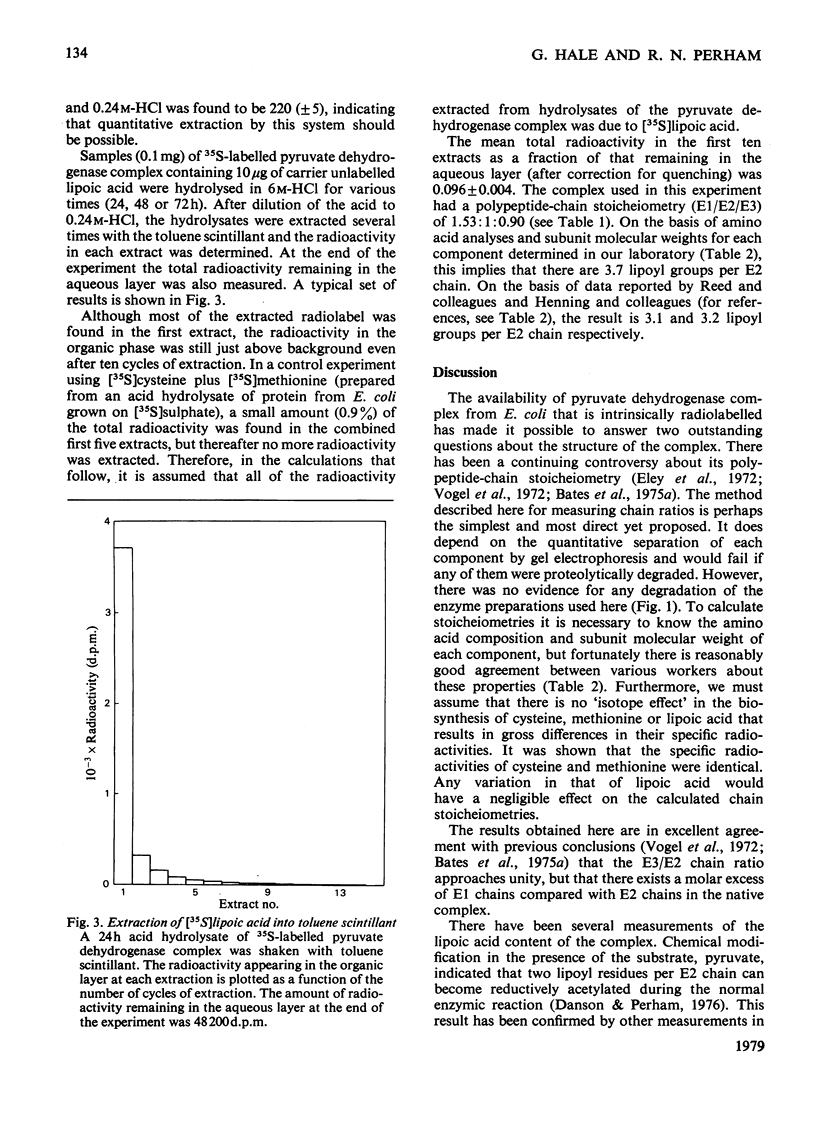


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates D. L., Danson M. J., Hale G., Hooper E. A., Perham R. N. Self-assembly and catalytic activity of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Nature. 1977 Jul 28;268(5618):313–316. doi: 10.1038/268313a0. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Harrison R. A., Perham R. N. The stoichiometry of polypeptide chains in the pyruvate dehydrogenase multienzyme complex of E. coli determined by a simple novel method. FEBS Lett. 1975 Dec 15;60(2):427–430. doi: 10.1016/0014-5793(75)80764-2. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Coggins J. R., Hooper E. A., Perham R. N. Use of dimethyl suberimidate and novel periodate-cleavable bis(imido esters) to study the quaternary structure of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochemistry. 1976 Jun 15;15(12):2527–2533. doi: 10.1021/bi00657a006. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Reed L. J. Acyl group and electron pair relay system: a network of interacting lipoyl moieties in the pyruvate and alpha-ketoglutarate dehydrogenase complexes from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4223–4227. doi: 10.1073/pnas.74.10.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J., Hooper E. A., Perham R. N. Intramolecular coupling of active sites in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1978 Oct 1;175(1):193–198. doi: 10.1042/bj1750193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J., Perham R. N. Evidence for two lipoic acid residues per lipoate acetyltransferase chain in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1976 Dec 1;159(3):677–682. doi: 10.1042/bj1590677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchschlag H. X-ray small-angle studies of the pyruvate dehydrogenase core complex from Escherichia coli K-12. II. Subunit structure of the core complex. Biophys Struct Mech. 1975 May 30;1(3):169–188. doi: 10.1007/BF00535755. [DOI] [PubMed] [Google Scholar]
- Eley M. H., Namihira G., Hamilton L., Munk P., Reed L. J. -Keto acid dehydrogenase complexes. 18. Subunit composition of the Escherichia coli pyruvate dehydrogenase complex. Arch Biochem Biophys. 1972 Oct;152(2):655–669. doi: 10.1016/0003-9861(72)90262-7. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. I. Purification and properties of pyruvate and alpha-ketoglutarate dehydrogenation complexes of Escherichia coli. J Biol Chem. 1960 Jul;235:1924–1930. [PubMed] [Google Scholar]
- Mitra S. K., Mandal R. K., Burma D. P. A biosynthetic method for preparing 35S-labelled lipoic acid. Biochim Biophys Acta. 1965 Aug 24;107(1):131–133. doi: 10.1016/0304-4165(65)90398-3. [DOI] [PubMed] [Google Scholar]
- Moe O. A., Jr, Lerner D. A., Hammes G. G. Fluorescence energy transfer between the thiamine diphosphate and flavine adenine dinucleotide binding sites on the pyruvate dehydrogenase multienzyme complex. Biochemistry. 1974 Jun 4;13(12):2552–2557. doi: 10.1021/bi00709a012. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Hooper E. A. Polypeptide chain stoicheiometry in the self-assembly of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. FEBS Lett. 1977 Feb 1;73(2):137–140. doi: 10.1016/0014-5793(77)80965-4. [DOI] [PubMed] [Google Scholar]
- Perham R. N. Self-assembly of biological macromolecules. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):123–136. doi: 10.1098/rstb.1975.0075. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. The subunit molecular weights of the alpha-ketoacid dehydrogenase multienzyme complexes from E. coli. FEBS Lett. 1971 Jun 2;15(1):8–12. doi: 10.1016/0014-5793(71)80066-2. [DOI] [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- Reed L. J., Pettit F. H., Eley M. H., Hamilton L., Collins J. H., Oliver R. M. Reconstitution of the Escherichia coli pyruvate dehydrogenase complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3068–3072. doi: 10.1073/pnas.72.8.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI K., REED L. J. LIPOAMIDASE. J Biol Chem. 1963 Dec;238:4021–4025. [PubMed] [Google Scholar]
- Shepherd G. B., Hammes G. G. Fluorescence energy transfer measurements in the pyruvate dehydrogenase multienzyme complex from Escherichia coli with chemically modified lipoic acid. Biochemistry. 1977 Nov 29;16(24):5234–5241. doi: 10.1021/bi00643a012. [DOI] [PubMed] [Google Scholar]
- Speckhard D. C., Ikeda B. H., Wong S. S., Frey P. A. Acetylation stoichiometry of Escherichia coli pyruvate dehydrogenase complex. Biochem Biophys Res Commun. 1977 Jul 25;77(2):708–713. doi: 10.1016/s0006-291x(77)80036-3. [DOI] [PubMed] [Google Scholar]
- Vogel O., Beikirch H., Müller H., Henning U. The subunit structure of the Escherichia coli K-12 pyruvate dehydrogenase complex. The dihydrolipoamide transacetylase component. Eur J Biochem. 1971 May 28;20(2):169–178. doi: 10.1111/j.1432-1033.1971.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Vogel O., Henning U. Pyruvate dehydrogenase component subunit structure of the Escherichia coli K 12 pyruvate dehydrogenase complex. Eur J Biochem. 1971 Jan 1;18(1):103–115. doi: 10.1111/j.1432-1033.1971.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Vogel O., Henning U. The subunit structure of the Escherichia coli K12 pyruvate-dehydrogenase complex. Dihydrolipoamide-dehydrogenase component. Eur J Biochem. 1973 Jun;35(2):307–310. doi: 10.1111/j.1432-1033.1973.tb02839.x. [DOI] [PubMed] [Google Scholar]
- Vogel O., Hoehn B., Henning U. Molecular structure of the pyruvate dehydrogenase complex from Escherichia coli K-12. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1615–1619. doi: 10.1073/pnas.69.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel O. Redetermination of the molecular weights of the components of the pyruvate dehydrogenase complex from E. coli Kl2+. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1235–1241. doi: 10.1016/0006-291x(77)91650-3. [DOI] [PubMed] [Google Scholar]
- Yanagawa H., Egami F. Asparagusate dehydrogenases and lipoyl dehydrogenase from asparagus mitochondria. Physical, chemical, and enzymatic properties. J Biol Chem. 1976 Jun 25;251(12):3637–3644. [PubMed] [Google Scholar]



