Abstract
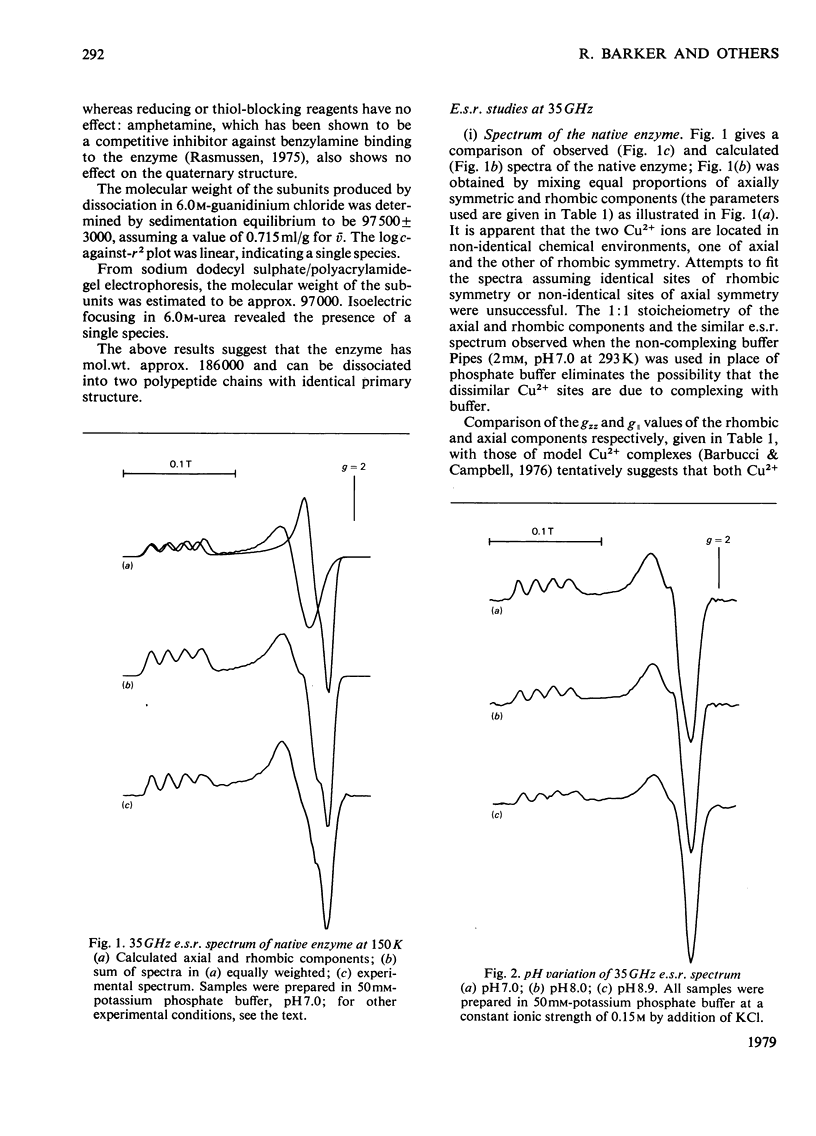
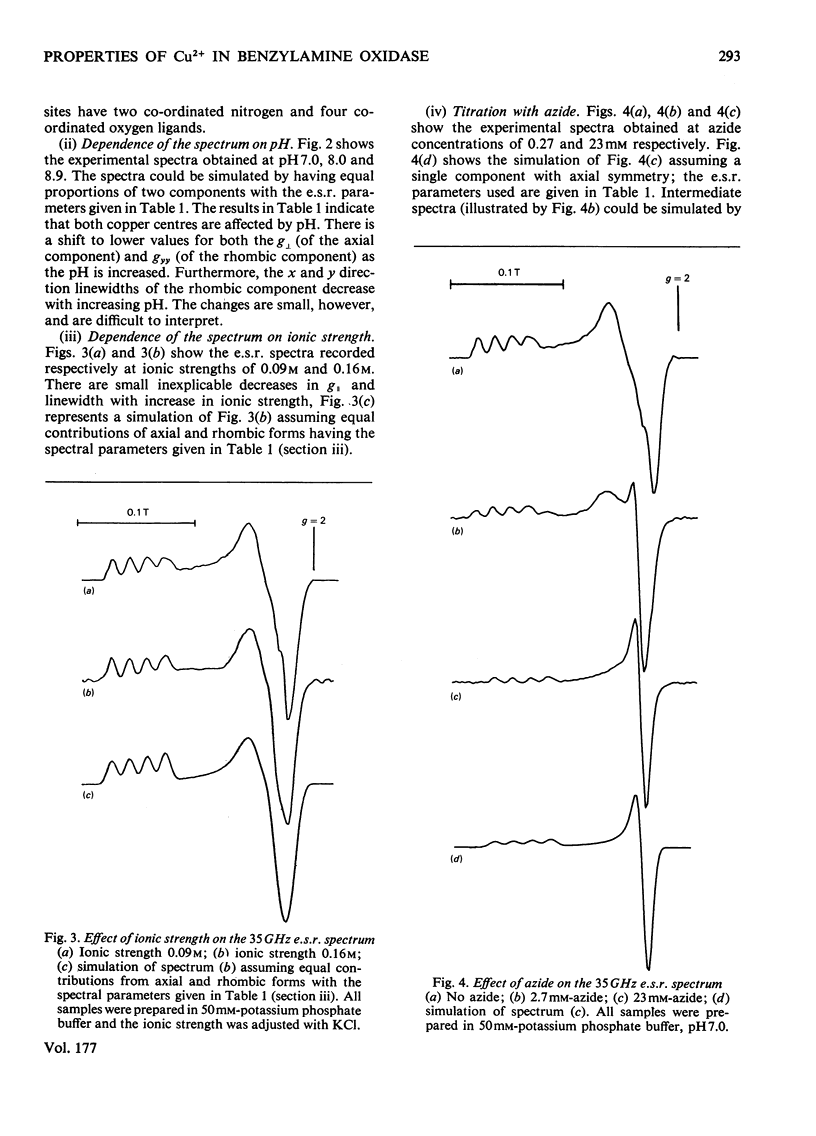
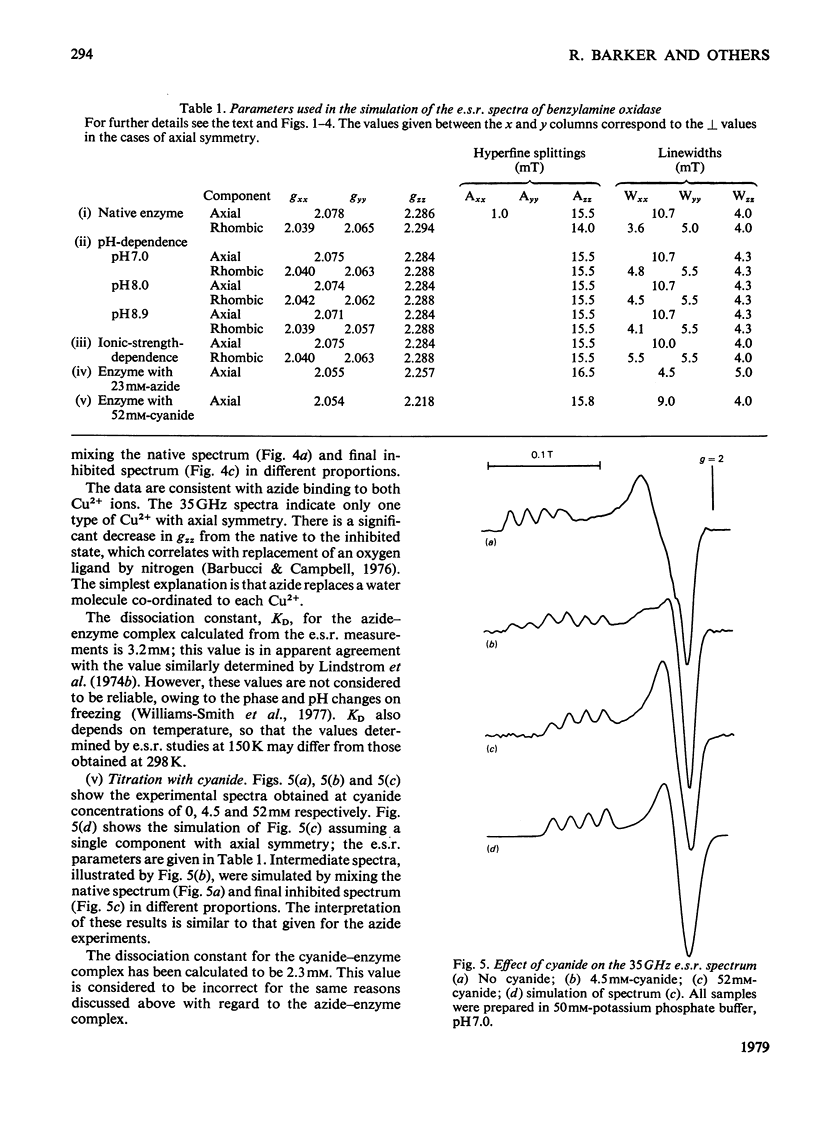
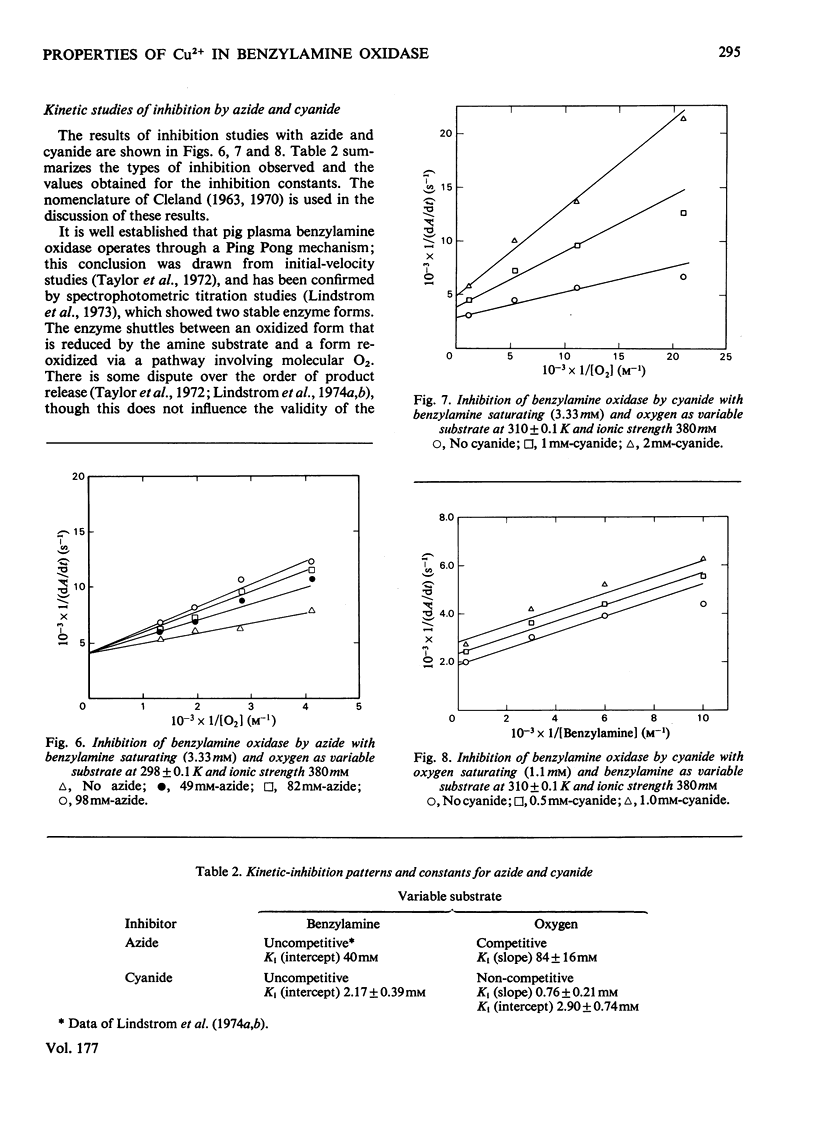
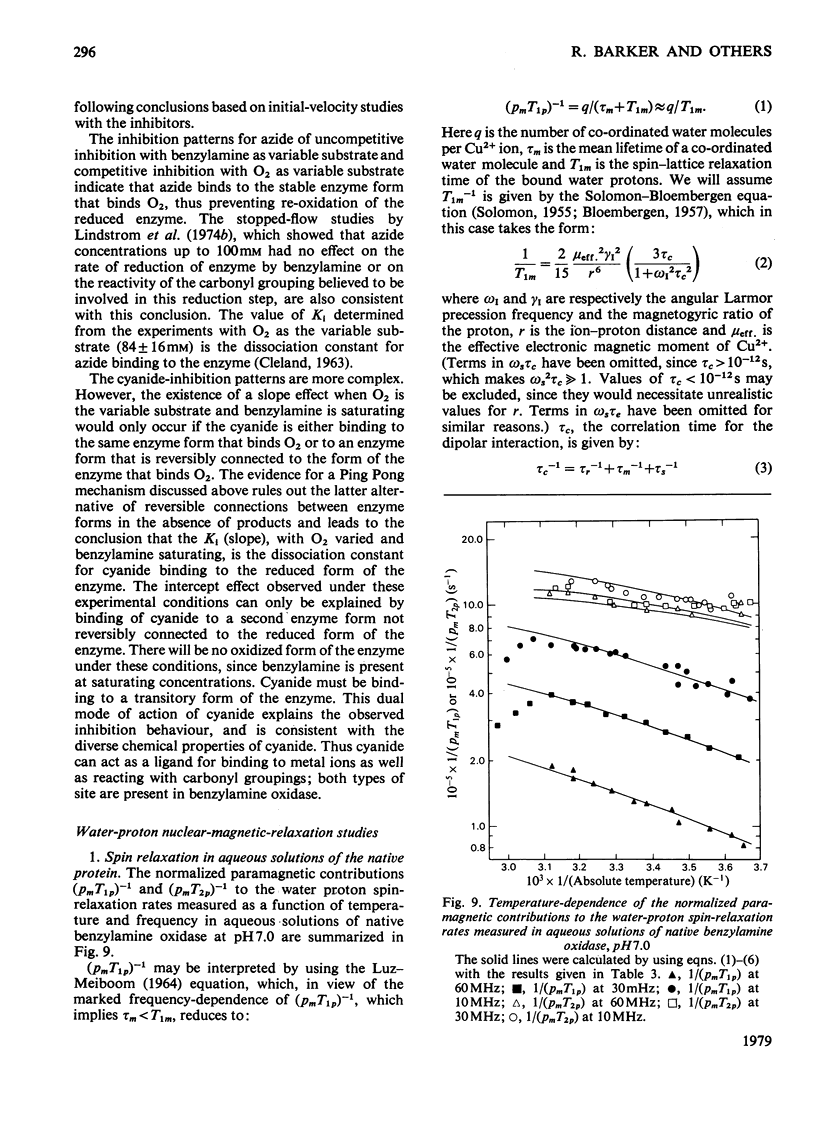
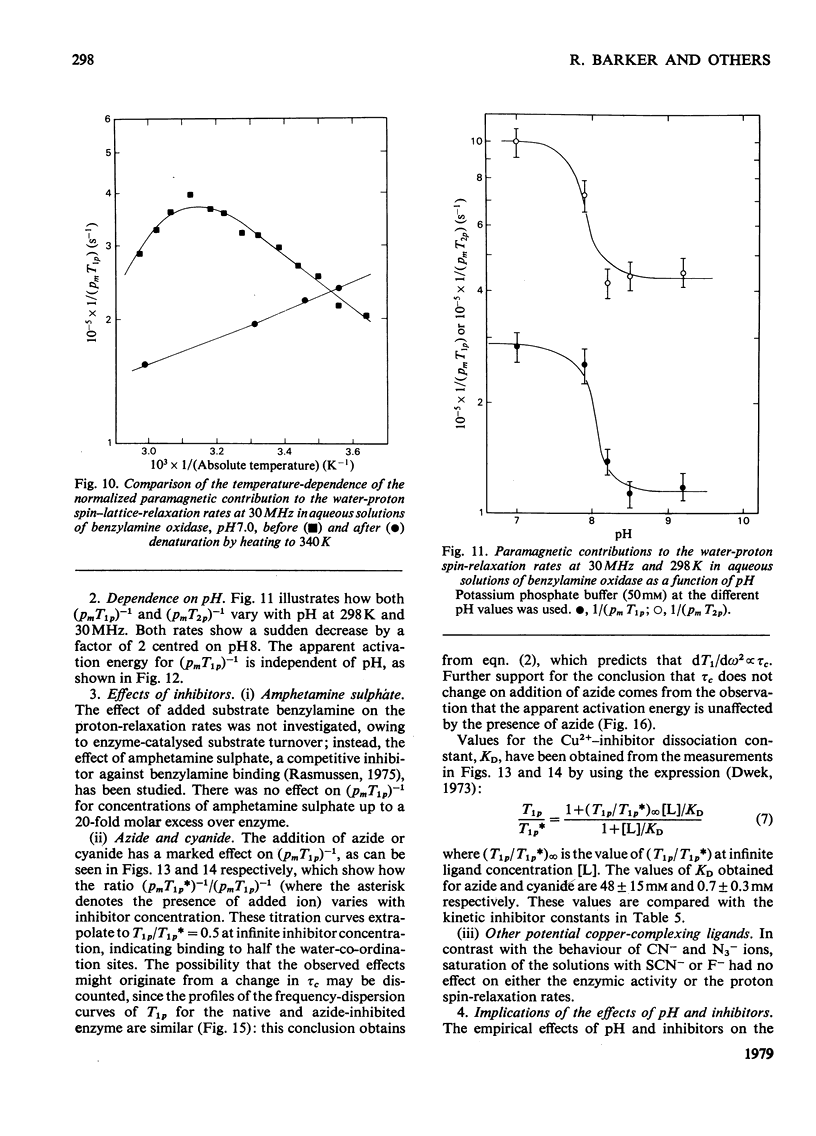
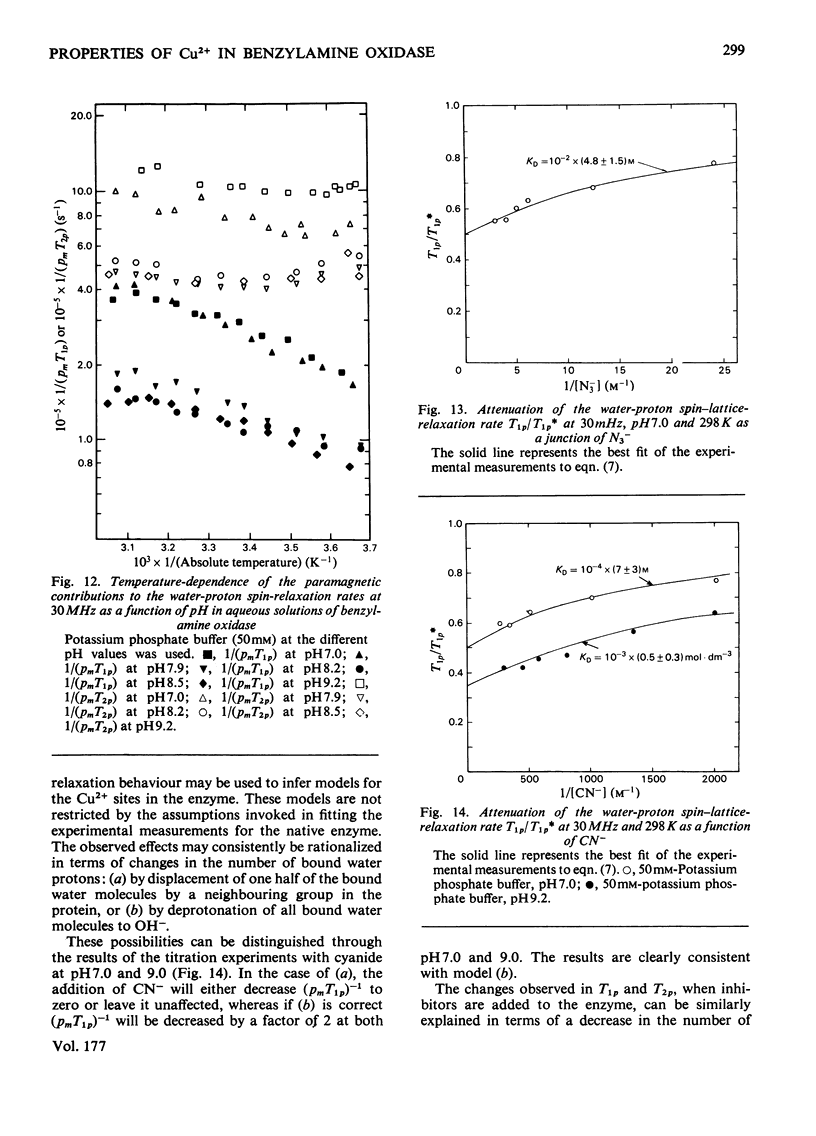
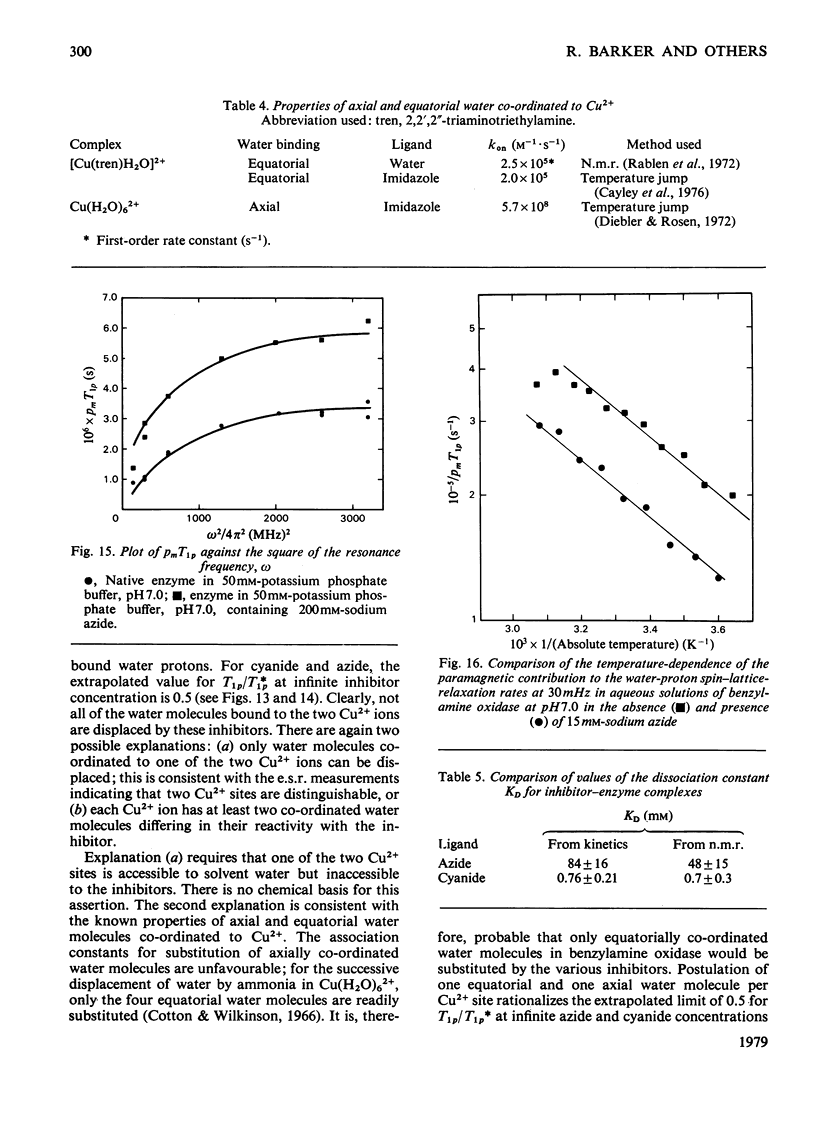
Benzylamine oxidase from pig plasma has been studied by a variety of chemical and physical techniques. 1. Analytical ultracentrifugation, gel electrophoresis and isoelectric-focusing studies suggest that the enzyme is composed of two subunits with closely similar primary structures. 2. E.s.r. and n.m.r. measurements show that the enzyme contains two well-separated (greater than 0.6 nm) Cu2+ ions at chemically distinct sites. Each Cu2+ ion is coordinated by two water molecules, one 'axial' and the other 'equatorial'. Both water molecules undergo fast exchange (10(5)--10(8) s-1) with solvent and are deprotonated in the pH range 8--9, but only the equatorial water molecule is displaced by the inhibitors N3- and CN-. 3. Kinetic and e.s.r. measurements show that azide and cyanide compete against O2 binding and also make the two Cu2+ sites identical. It is concluded that Cu2+ must participate in the re-oxidation of reduced enzyme by molecular O2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLASCHKO H., BUFFONI F. PYRIDOXAL PHOSPHATE AS A CONSTITUENT OF THE HISTAMINASE (BENZYLAMINE OXIDASE) OF PIG PLASMA. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:45–60. doi: 10.1098/rspb.1965.0059. [DOI] [PubMed] [Google Scholar]
- BUFFONI F., BLASCHKO H. BENZYLAMINE OXIDASE AND HISTAMINASE: PURIFICATION AND CRYSTALLIZATION OF AN ENZYME FROM PIG PLASMA. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:153–167. doi: 10.1098/rspb.1964.0086. [DOI] [PubMed] [Google Scholar]
- Boden N., Holmes M. C., Knowles P. F. Binding of water to "types I and II" Cu2+ in proteins. Biochem Biophys Res Commun. 1974 Apr 8;57(3):845–848. doi: 10.1016/0006-291x(74)90623-8. [DOI] [PubMed] [Google Scholar]
- Boden N., Holmes M. C., Knowles P. F. Properties of the cupric sites in bovine superoxide dismutase studied by nuclear-magnetic-relaxation measurements. Biochem J. 1979 Jan 1;177(1):303–309. doi: 10.1042/bj1770303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F., Corte L. D., Knowles P. F. The nature of copper in pig plasma benzylamine oxidase. Biochem J. 1968 Jan;106(2):575–576. doi: 10.1042/bj1060575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F., Ignesti G. Active-sitve titration of pig plasma benzylamine oxidase with phenylhydrazine. Biochem J. 1975 Feb;145(2):369–372. doi: 10.1042/bj1450369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Curzon G. The inhibition of caeruloplasmin by azide. Biochem J. 1966 Aug;100(2):295–302. doi: 10.1042/bj1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein S. J., Schachman H. K. The simultaneous determination of partial specific volumes and molecular weights with microgram quantities. J Biol Chem. 1967 Jan 25;242(2):306–311. [PubMed] [Google Scholar]
- Fletterick R. J., Bates D. J., Steitz T. A. The structure of a yeast hexokinase monomer and its complexes with substrates at 2.7-A resolution. Proc Natl Acad Sci U S A. 1975 Jan;72(1):38–42. doi: 10.1073/pnas.72.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström A., Olsson B., Petterson G. Effect of azide on some spectral and kinetic properties of pig-plasma benzylamine oxidase. Eur J Biochem. 1974 Oct 1;48(1):237–243. doi: 10.1111/j.1432-1033.1974.tb03761.x. [DOI] [PubMed] [Google Scholar]
- Lindström A., Olsson B., Pettersson G. Kinetics of the interaction between pig-plasma benzylamine oxidase and substrate. Eur J Biochem. 1973 May;35(1):70–77. doi: 10.1111/j.1432-1033.1973.tb02811.x. [DOI] [PubMed] [Google Scholar]
- Lindström A., Olsson B., Pettersson G. Transient kinetics of benzaldehyde formation during the catalytic action of pig-plasma benzylamine oxidase. Eur J Biochem. 1974 Mar 1;42(2):377–381. doi: 10.1111/j.1432-1033.1974.tb03349.x. [DOI] [PubMed] [Google Scholar]
- Lindström A., Pettersson G. Active-site titration of pig-plasma benzylamine oxidase with hydrazine derivatives. Eur J Biochem. 1973 May 2;34(3):564–568. doi: 10.1111/j.1432-1033.1973.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Lindström A., Pettersson G. The mechanism of inhibition of pig-plasma benzylamine oxidase by the copper-chelating reagent cuprizone. Eur J Biochem. 1974 Oct 1;48(1):229–236. doi: 10.1111/j.1432-1033.1974.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Neumann R., Hevey R., Abeles R. H. The action of plasma amine oxidase on beta-haloamines. Evidence for proton abstraction in the oxidative reaction. J Biol Chem. 1975 Aug 25;250(16):6362–6367. [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. Kinetic isotope effects on the catalytic activity of pig-plasma benzylamine oxidase. Eur J Biochem. 1976 May 1;64(2):327–331. doi: 10.1111/j.1432-1033.1976.tb10305.x. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Taylor C. E., Taylor R. S., Rasmussen C., Knowles P. F. A catalytic mechanism for the enzyme benzylamine oxidase from pig plasma. Biochem J. 1972 Dec;130(3):713–728. doi: 10.1042/bj1300713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Bogart M., Beinert H. Micro methods for the quantitative determination of iron and copper in biological material. Anal Biochem. 1967 Aug;20(2):325–334. doi: 10.1016/0003-2697(67)90038-3. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yasunobu K. T. Carbohydrate content of bovine plasma amine oxidase and isolation of a carbohydrate-containing fragment attached to asparagine. J Biol Chem. 1970 Sep 25;245(18):4612–4617. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams-Smith D. L., Bray R. C., Barber M. J., Tsopanakis A. D., Vincent S. P. Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem J. 1977 Dec 1;167(3):593–600. doi: 10.1042/bj1670593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


