Abstract
Background
Sensory impairments (SI), including vision (VI), hearing (HI), and dual sensory impairments (DSI), are prevalent with aging, but their impact on disease risk remains unclear. This study investigates the epidemiological and genetic associations between SIs and 10 chronic disease categories and multimorbidity.
Methods
Using the CHARLS study, participants were classified by their self-reported VI/HI/DSI status in 2011 and 2013 into groups: “new onset, remission, persistent, and no SI.” Their chronic disease incidence was tracked until 2018 in sub-cohorts respectively. Mendelian randomization (MR) analyses used genetic instruments from UK Biobank GWAS data on 88,250/504,307 individuals for vision/hearing loss, with outcome datasets from consortia including FinnGen, DIAMANTE, CKDGen, PGC, GWAS Catalog, and International Parkinson’s Disease Genomics Consortium.
Results
The cohort study revealed that persistent HI significantly increased the risk of heart disease (P < 0.001, HR 1.63, 95% CI 1.31–2.03), stroke (P 0.004, HR 1.59, 95% CI 1.16–2.18), chronic lung disease (P 0.002, HR 1.53, 95% CI 1.17–1.99), and emotional, nervous, or psychiatric problems (P 0.016, HR 2.03, 95% CI 1.14–3.60). Persistent VI was significantly associated with diabetes or high blood sugar (DM/Hglu) (P 0.012, HR 1.63, 95% CI 1.11–2.38) and chronic lung disease (P 0.042, HR 1.53, 95% CI 1.02–2.31). MR confirmed these strong or suggestive associations, indicating that HI significantly increased the risk of cardiovascular and cerebrovascular events by 61–170%, bronchitis by 160%, and schizophrenia by 36%. In addition, VI significantly raised the risk of hyperglycemia or diabetes by 2–4% and the risk of lung function decline.
Additionally, cohort studies confirmed that early DSI significantly raised the risk of multiple diseases, while MR identified genetic links between VI and hepatic failure, Parkinson’s, and Alzheimer’s disease, and between HI and hypertension, chronic kidney disease, and renal failure.
Conclusions
This study provides evidence from epidemiological or genetic perspectives demonstrates that early exposure to HI/VI/DSI increases the risk of developing chronic diseases. These findings underscore the need for continuous monitoring and timely intervention for SI to manage chronic disease risks in aging populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-025-03857-x.
Keywords: Vision impairment (VI), Hearing impairment (HI), Dual sensory impairment (DSI), Chronic diseases, Multimorbidity, Cohort study (CS), Mendelian randomization analyses (MR)
Background
Vision and hearing are essential for individuals to perceive their environment, communicate effectively, and engage in social activities, which are crucial for daily life and independence. According to the World Report on Vision/Hearing, in 2019, at least 2.2 billion people globally were affected by vision impairments (VI) and 1.5 billion by hearing impairments (HI) [1], with these populations rapidly expanding [2, 3]. Beyond being major causes of disability, VI&HI significantly impact quality of life and psychological well-being [4]. Recent epidemiological studies have highlighted their intersection with chronic diseases [5–9]. However, these studies often focus on the co-occurrence of conditions or view sensory impairments as outcomes of chronic diseases [6], rather than investigating their prospective impact on disease development. This approach neglects the potential of sensory impairments as early indicators or independent risk factors that could predict and exacerbate chronic disease progression.
VI and HI range from mild losses to complete blindness and deafness. Self-reported changes in sensory status may more comprehensively reflect biological associations with disease risk than single-time reports. Early identification and intervention of sensory impairments through vision and hearing care services can potentially halt or reverse the progression of sensory loss. Assessing early changes in vision and hearing provides critical evidence for proactive monitoring and intervention in managing chronic diseases and multimorbidity in middle-aged and older adults.
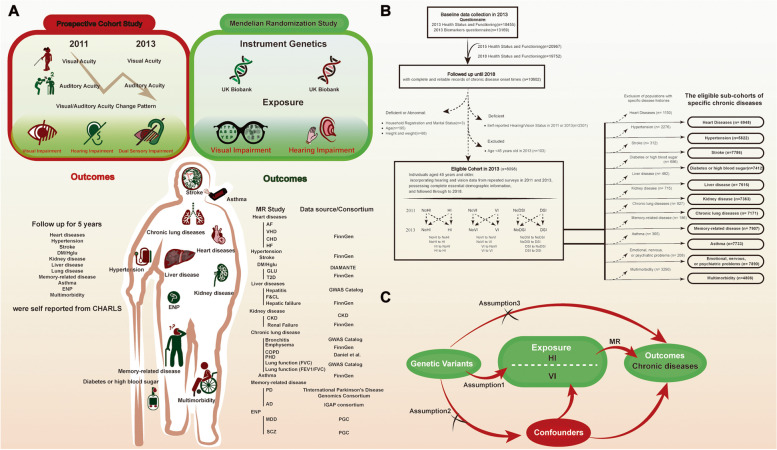
This study uses a prospective design to evaluate how early sensory impairments affect the risk of chronic diseases. By repeatedly measuring changes in VI, HI, and dual sensory impairment (DSI) over time, the study assesses specific risk effects on diseases. Genome-wide association studies (GWAS) have identified genetic variants associated with VI and HI [10, 11], enabling the use of Mendelian randomization (MR) to clarify the causal relationships between sensory loss and chronic diseases. Specific genetic markers associated with HI and VI serve as instrumental variables to estimate causal effects on a range of chronic disease outcomes (Fig. 1A).
Fig. 1.
Overview and detailed workflow diagram of the study design. A Conceptual Diagram: Investigating the Impact of Baseline Exposure to Vision and Hearing Impairments on Chronic Disease Risk Using Prospective Cohort and Mendelian Randomization Studies. B Screening of the study population and establishment of 11 sub-cohorts (10 chronic diseases and multimorbidity) 1Dataset of “Biomarker” includes information of participants’ weight, height, grip strength and physical fitness etc. 2Dataset of “Health_status_and_function ” includes records of participants’ chronic disease onset time, auditory acuity, distance visual acuity, near visual acuity and etc. C Diagram of Mendelian Randomization Assumptions in This Study. Three basic assumptions are required for the genetic variants to qualify as valid instrumental variables (IVs): (Assumption1) they should be robustly associated with the exposure; (Assumption2) they should not be associated with potential confounders of the exposure-outcome association; and (Assumption3) they should not influence the outcome by any variable other than the exposure
Methods
Prospective cohort study (CS) design
Population
The study population was derived from the China Health and Retirement Longitudinal Study (CHARLS). CHARLS is a nationwide survey conducted in four stages (county, village, household, individual) and collects comprehensive data on health, psychological and cognitive status, lifestyle, etc. Beginning in 2011, CHARLS tracks and follows up with the population every 2 years.
To clarify the effects of early VI&HI&DSI and their dynamic changes on the risk of chronic diseases and multimorbidity in populations, we established 11 sub-cohorts for 10 chronic diseases and multimorbidity (excluding individuals with a history of the specific disease at baseline in 2013) and followed them up until 2018.
The inclusion criteria for the cohort population in this study are as follows:
Participants who reported health status (n = 18,455) and provided biomarker data (n = 13,169) in 2013.
Participants who were followed up in 2015 (n = 20,967) and 2018 (n = 19,752) with health status questionnaires.
Furthermore, participants were excluded based on the following criteria:
Missing data on age (n = 195), household registration status (n = 3), or height and weight (n = 88).
Age below 45 years (n = 103).
No valid reports of both hearing and vision levels for 2011 and 2013 (n = 2301).
For each specific disease sub-cohort, participants reporting a history of the disease at baseline (2013).
The occurrence and specific timing of target outcomes for every individual in each cohort were recorded. The study design and the population selection flowchart are shown in Fig. 1B.
Exposure: evaluation of HI, VI, DSI, and classification of dynamic transition patterns
Respondents’ hearing and vision status were obtained through face-to-face questionnaires:
Hearing Status: “DA039 Is your hearing very good, good, fair, poor, or very poor (with a hearing aid if you normally use it and without if you normally don’t)? Would you say your hearing is excellent, very good, good, fair, or poor?”.
Vision status
Distance Vision: “DA033 How good is your eyesight for seeing things at a distance, like recognizing a friend from across the street (with glasses or corrective lenses if you wear them)? Would you say your eyesight for seeing things at a distance is excellent, very good, good, fair, or poor?”.
Near Vision: “How good is your eyesight for seeing things up close, like reading ordinary newspaper print (with glasses or corrective lenses if you wear them)? Would you say your eyesight for seeing things up close is excellent, very good, good, fair, or poor?”.
Respondents self-evaluated their hearing and vision on a scale of 1. Excellent, 2. Very good, 3. Good, 4. Fair, 5. Poor.
Impairment was defined as “fair” or “poor.”
HI: Present if a response of “fair” or “poor” to the corresponding question.
VI: Present if either distance or near vision impairment was reported.
DSI: Present if both hearing and vision impairments were reported.
HI/VI/DSI transition patterns
Combining the HI/VI/DSI reports from 2011 and 2013, we classified four transition patterns:
HI/VI/DSI transition patterns
No HI/VI/DSI–No HI/VI/DSI: Never HI/VI/DSI (no HI/VI/DSI in both 2011 and 2013).
No HI/VI/DSI–HI/VI/DSI: Newly developed HI/VI/DSI (no HI/VI/DSI in 2011, developed HI/VI/DSI in 2013).
HI/VI/DSI–No HI/VI/DSI: Relieved HI/VI/DSI (HI/VI/DSI in 2011, no HI/VI/DSI in 2013).
HI/VI/DSI–HI/VI/DSI: Persistent HI/VI/DSI (HI/VI/DSI in both 2011 and 2013).
Outcomes: evaluation of 13 chronic diseases and multimorbidity
The identification of 10 chronic diseases (whether present and the first diagnosis time) was conducted through face-to-face questionnaires: “DA007 Have you been diagnosed with [conditions listed below, read one by one] by a doctor?” and “DA009 When was the condition first diagnosed or known by yourself? ___year or ___age?” Multimorbidity was defined as the presence of two or more chronic diseases.
When the onset time was 2013 or earlier, it was considered a pre-existing condition; when the onset time was after 2013, it was considered a new incident during follow-up.
Additionally, the right-censoring criterion in this study is defined by the end of the follow-up period, specifically the conclusion of the fourth wave of the survey in 2018 (at the end of September 2018). Positive outcome events for each sub-disease cohort were defined as the first occurrence of the reported disease during the follow-up period.
Covariates
Covariates included age, gender, BMI, household registration type, and marital status. Smoking and drinking status were obtained through questionnaires: “DA059 Have you ever chewed tobacco, smoked a pipe, smoked self-rolled cigarettes, or smoked cigarettes/cigars?” and “DA069 Did you ever drink alcoholic beverages in the past? How often?”.
Social and individual activity levels were assessed through the question “Have you done any of these activities in the last month?” Social activity levels were evaluated based on participation in “socializing with friends,” “participating in clubs,” “engaging in organizations,” and “volunteering.” Personal activity levels were evaluated based on participation in “playing Mahjong,” “attending courses,” “stock trading,” and “surfing the internet.” Activities were classified as “inactive,” “moderately active,” and “active” based on monthly participation frequency.
Physical fitness was assessed using the “five-time sit-to-stand” test, with “low physical fitness” defined as an inability to complete the test or taking more than 12 s.
Grip strength was evaluated using the average of multiple measurements, with “low grip strength” defined as an average grip strength below 28 kg for men or below 18 kg for women.
Additionally, the history of hypertension, hyperlipidemia, and diabetes or high blood sugar (DM/Hglu) were included as covariates.
MR
The study employed a series of two-sample MR methods using summary data from GWAS. The exposures analyzed by MR included VI caused by five ocular diseases and self-reported HI [7, 8], with instrumental variables derived from the UK Biobank (UKB) GWAS. This research applied 24 chronic diseases as outcomes in the study, including atrial fibrillation and flutter (AF) [12], valvular heart disease (VHD) [12], major coronary heart disease event (CHD) [12], heart failure (HF) [12], hypertension [12], stroke [12], blood glucose levels (GLU) [13], type 2 diabetes (T2D) [14], hepatitis [13], fibrosis and cirrhosis of liver (F&CL) [12], hepatic failure [12], chronic kidney disease (CKD) [15], renal failure [12], bronchitis [13], emphysema [12], chronic obstructive pulmonary disease (COPD) [12], pulmonary heart disease (PHD) [16], lung function (FVC [13]; FEV1/FVC [13]), Parkinson’s disease (PD) [17], Alzheimer’s disease (AD), asthma [12], major depressive disorders (MDD) [18], and schizophrenia (SCZ) [19]. The data were obtained from various public GWAS resources, including FinnGen consortium (https://r10.finngen.fi/), the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), the GWAS Catalog (https://www.ebi.ac.uk/gwas/), as well as other disease-specific consortia such as the Diabetes Meta-Analysis of Trans-Ethnic Association Studies (DIAMANTE) Consortium, the CKDGen Consortium, the Psychiatric Genomic Consortium (PGC), the International Genomics of Alzheimer’s Project (IGAP) consortium, and International Parkinson’s Disease Genomics Consortium (Fig. 1A, C). Detailed information on the MR design is provided in Additional File1: Table S1.
Statistical analyses
To test the differences in baseline characteristics across HI, VI, and DSI transition patterns, ANOVA was used for normally distributed continuous variables, and chi-square tests were used for categorical variables. Cox proportional hazards regression models were employed to evaluate the risk effects of hearing/vision levels, HI/VI/DSI, and their transition patterns on outcomes (new chronic diseases and multimorbidity), reported as adjusted hazard ratios (aHR) with 95% confidence intervals (CI) and presented in forest plots. The Cox proportional hazards models were tested for major violations of assumptions. Log-rank test-based Kaplan–Meier analysis was used to present the cumulative incidence of new outcomes within the 11 sub-cohorts based on HI/VI/DSI groups. Additionally, population attributable fractions (PAF) and their CIs were calculated, adjusted for covariates, to quantify the burden of chronic diseases and multimorbidity attributable to HI/VI/DSI and their dynamic changes.
In sensitivity analyses, we repeated the primary analyses of the risk effects of HI/VI/DSI and their transition patterns on new chronic diseases and multimorbidity: (1) multiple imputation for missing data using chained equations; (2) propensity score matching (PSM) for overlapping weighting of confounding factors; (3) excluding individuals who developed the disease within the first year. All models were adjusted for age, gender, BMI, household registration status, marital status, smoking and drinking habits, social and individual activity levels, low physical fitness, low grip strength, and history of hypertension, hyperlipidemia, and DM/Hglu.
For MR analysis, the inverse-variance weighted (IVW) method is utilized as the primary approach [20]. This method operates under the assumption that pleiotropy is either absent or balanced. Additionally, supplementary methods MR-Egger and the weighted-median were employed alongside IVW. The MR-Egger method is reliable when more than 50% of the IVs are subject to horizontal pleiotropy [21].The weighted-median method assumes that most IVs are valid and is considered robust when the percentage of horizontal pleiotropic IVs is < 50% [22]. To ensure the reliability of the final analysis results, the study employed specific screening criteria as filters for robust significant causality. These criteria included: (1) At least IVW method suggested a significant causal relationship. (2) Consistency in the direction of MR analysis results (β value) across all three methods.
The Bonferroni correction for multiple testing was conducted to correct P values [23]. A P value less than 1.04E − 03 (0.05/24/2 = 0.00104) was considered as strong evidence of a causal association. A P value falling between 1.04E − 03 and 0.05 was considered as suggestive evidence for a potential causal association. Meanwhile, the corresponding 99.896% (1–0.00104) CI was calculated based on the Bonferroni-corrected α_adjusted (0.00104) and reported alongside the conventional 95% CI. We assessed heterogeneity using Cochran’s Q test [24]. In addition, MR‐Egger intercept and MRPRESSO were used to evaluate potential horizontal pleiotropy [25, 26]. If the outliers were detected, they would be removed, and the MR causal estimation would be reassessed. The outcomes corrected by MRPRESSO were included in the primary results, thereby expanding the IVW analysis framework. Finally, “leave-one-out” analyses were performed to evaluate whether any single SNP was driving the results [27].All MR analyses were performed out in R software 2023.12.1 + 402 with “TwoSampleMR”, “MRPRESSO”packages.
Results
Baseline characteristics
Significant differences in demographic characteristics were observed across groups of NoHI-NoHI, HI-NoHI, NoHI-HI, and HI-HI: individuals with persistent no HI were the youngest on average, had the highest proportion of males, highest BMI levels, and the lowest proportion of agricultural household registration. Additionally, they had the lowest proportion of non-drinkers, greater participation in social and personal activities, and fewer individuals with low physical fitness and low grip strength. At baseline, individuals with persistent HI had the highest number of comorbidities and a higher prevalence of 11 chronic diseases, including heart disease and hyperlipidemia, as well as a higher rate of multimorbidity.
Similarly, self-reported VI over 2 years exhibited four patterns: persistent no VI, transition from VI to no VI, transition from no VI to VI, and persistent VI. Individuals with persistent no VI were younger, had a higher proportion of males, higher BMI levels, and the highest rates of smoking.
In contrast, those with persistent VI had lower social and personal activity levels and a higher number of comorbidities at baseline. This group also had the significantly highest prevalence of 11 chronic diseases and multimorbidity, except for memory-related diseases (Table 1).
Table 1.
Stratified baseline characteristics of participants according to the VI/HI transition patterns from 2011 to 2013
| NoVI-NoVI | NoVI-VI | VI-NoVI | VI-VI | p | NoHI-NoHI | NoHI-HI | HI-NoHI | HI-HI | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 644 | 1135 | 922 | 5397 | 1996 | 1615 | 1063 | 3424 | |||
| Age | 58.79 (9.32) | 58.94 (9.00) | 60.84 (8.93) | 60.66 (8.62) | < 0.001 | 58.43 (8.45) | 59.58 (8.61) | 60.35 (8.59) | 61.69 (8.91) | < 0.001 | |
| Sex = male (%) | 356 (55.3) | 554 (48.8) | 453 (49.1) | 2329 (43.2) | < 0.001 | 949 (47.5) | 743 (46.0) | 435 (40.9) | 1565 (45.7) | 0.006 | |
| BMI (kg/m2) | 24.31 (3.68) | 23.92 (3.79) | 23.81 (3.56) | 23.85 (3.85) | 0.03 | 24.15 (3.85) | 23.79 (3.77) | 24.01 (3.86) | 23.76 (3.76) | 0.002 | |
| Residence Registration Status = agricultural (%) | 509 (79.0) | 906 (79.8) | 734 (79.6) | 4480 (83.0) 0.002 | 1550 (77.7) | 1331 (82.4) | 896 (84.3) | 2852 (83.3) < 0.001 | |||
| Marital Status = married (%) | 564 (87.6) | 1020 (89.9) | 801 (86.9) | 4755 (88.1) 0.183 | 1771 (88.7) | 1441 (89.2) | 933 (87.8) | 2995 (87.5) 0.252 | |||
| Smoke = yes (%) | 295 (45.8) | 474 (41.8) | 408 (44.3) | 2133 (39.5) 0.002 | 834 (41.8) | 659 (40.8) | 394 (37.1) | 1423 (41.6) 0.052 | |||
| Drink | < 0.001 | 0.019 | |||||||||
| = sometimes (%) | 190 (29.5) | 333 (29.3) | 270 (29.3) | 1313 (24.3) | 571 (28.6) | 436 (27.0) | 253 (23.8) | 846 (24.7) | |||
| = often (%) | 54 (8.4) | 84 (7.4) | 81 (8.8) | 417 (7.7) | 164 (8.2) | 119 (7.4) | 86 (8.1) | 267 (7.8) | |||
| = no (%) | 400 (62.1) | 718 (63.3) | 571 (61.9) | 3667 (67.9) | 1261 (63.2) | 1060 (65.6) | 724 (68.1) | 2311 (67.5) | |||
| Level of Social Activity Engagement | 0.003 | < 0.001 | |||||||||
| = active (%) | 222 (34.5) | 354 (31.2) | 312 (33.8) | 1671 (31.0) | 716 (35.9) | 481 (29.8) | 327 (30.8) | 1035 (30.2) | |||
| = moderate (%) | 81 (12.6) | 193 (17.0) | 124 (13.4) | 706 (13.1) | 296 (14.8) | 239 (14.8) | 127 (11.9) | 442 (12.9) | |||
| = non-participatory (%) | 341 (53.0) | 588 (51.8) | 486 (52.7) | 3020 (56.0) | 984 (49.3) | 895 (55.4) | 609 (57.3) | 1947 (56.9) | |||
| Level of Individual Activity Engagement | < 0.001 | < 0.001 | |||||||||
| = active (%) | 81 (12.6) | 111 (9.8) | 101 (11.0) | 398 (7.4) | 224 (11.2) | 118 (7.3) | 96 (9.0) | 253 (7.4) | |||
| = moderate (%) | 110 (17.1) | 190 (16.7) | 136 (14.8) | 807 (15.0) | 366 (18.3) | 263 (16.3) | 139 (13.1) | 475 (13.9) | |||
| = non-participatory (%) | 453 (70.3) | 834 (73.5) | 685 (74.3) | 4192 (77.7) | 1406 (70.4) | 1234 (76.4) | 828 (77.9) | 2696 (78.7) | < 0.001 | ||
| Physical Fitness Level = low (%) | 113 (17.5) | 243 (21.4) | 227 (24.6) | 1354 (25.1) | < 0.001 | 382 (19.1) | 354 (21.9) | 285 (26.8) | 916 (26.8) | < 0.001 | |
| Grip Strength = low (%) | 99 (15.4) | 197 (17.4) | 178 (19.3) | 1130 (20.9) | 0.001 | 279 (14.0) | 311 (19.3) | 219 (20.6) | 795 (23.2) | < 0.001 | |
| Auditory Acuity in 2013 | < 0.001 | < 0.001 | |||||||||
| = excellent (%) | 42 (6.5) | 18 (1.6) | 50 (5.4) | 72 (1.3) | 126 (6.3) | 0 (0.0) | 56 (5.3) | 0 (0.0) | |||
| = very good (%) | 204 (31.7) | 168 (14.8) | 243 (26.4) | 529 (9.8) | 810 (40.6) | 0 (0.0) | 334 (31.4) | 0 (0.0) | |||
| = good (%) | 222 (34.5) | 267 (23.5) | 307 (33.3) | 937 (17.4) | 1060 (53.1) | 0 (0.0) | 673 (63.3) | 0 (0.0) | |||
| = fair (%) | 144 (22.4) | 545 (48.0) | 244 (26.5) | 2938 (54.4) | 0 (0.0) | 1410 (87.3) | 0 (0.0) | 2461 (71.9) | |||
| = poor (%) | 32 (5.0) | 137 (12.1) | 78 (8.5) | 921 (17.1) | 0 (0.0) | 205 (12.7) | 0 (0.0) | 963 (28.1) | |||
| Auditory Acuity in 2011 | < 0.001 | < 0.001 | |||||||||
| = excellent (%) | 20 (3.1) | 25 (2.2) | 13 (1.4) | 48 (0.9) | 70 (3.5) | 36 (2.2) | 0 (0.0) | 0 (0.0) | |||
| = very good (%) | 204 (31.7) | 291 (25.6) | 115 (12.5) | 468 (8.7) | 681 (34.1) | 397 (24.6) | 0 (0.0) | 0 (0.0) | |||
| = good (%) | 277 (43.0) | 528 (46.5) | 275 (29.8) | 1347 (25.0) | 1245 (62.4) | 1182 (73.2) | 0 (0.0) | 0 (0.0) | |||
| = fair (%) | 118 (18.3) | 234 (20.6) | 415 (45.0) | 2663 (49.3) | 0 (0.0) | 0 (0.0) | 947 (89.1) | 2483 (72.5) | |||
| = poor (%) | 25 (3.9) | 57 (5.0) | 104 (11.3) | 871 (16.1) | 0 (0.0) | 0 (0.0) | 116 (10.9) | 941 (27.5) | |||
| Distance Visual Acuity in 2013 | < 0.001 | < 0.001 | |||||||||
| = excellent (%) | 68 (10.6) | 23 (2.0) | 79 (8.6) | 64 (1.2) | 115 (5.8) | 34 (2.1) | 48 (4.5) | 37 (1.1) | |||
| = very good (%) | 266 (41.3) | 108 (9.5) | 329 (35.7) | 288 (5.3) | 443 (22.2) | 125 (7.7) | 214 (20.1) | 209 (6.1) | |||
| = good (%) | 310 (48.1) | 183 (16.1) | 514 (55.7) | 531 (9.8) | 607 (30.4) | 254 (15.7) | 272 (25.6) | 405 (11.8) | |||
| = fair (%) | 0 (0.0) | 648 (57.1) | 0 (0.0) | 2882 (53.4) | 564 (28.3) | 842 (52.1) | 332 (31.2) | 1792 (52.3) | |||
| = poor (%) | 0 (0.0) | 173 (15.2) | 0 (0.0) | 1632 (30.2) | 267 (13.4) | 360 (22.3) | 197 (18.5) | 981 (28.7) | |||
| Distance Visual Acuity in 2011 | < 0.001 | < 0.001 | |||||||||
| = Excellent (%) | 40 (6.2) | 37 (3.3) | 11 (1.2) | 28 (0.5) | 58 (2.9) | 36 (2.2) | 4 (0.4) | 18 (0.5) | |||
| = excellent (%) | 252 (39.1) | 368 (32.4) | 84 (9.1) | 265 (4.9) | 448 (22.4) | 259 (16.0) | 81 (7.6) | 181 (5.3) | |||
| = very good (%) | 352 (54.7) | 730 (64.3) | 174 (18.9) | 728 (13.5) | 710 (35.6) | 569 (35.2) | 193 (18.2) | 512 (15.0) | |||
| = good (%) | 0 (0.0) | 0 (0.0) | 508 (55.1) | 2747 (50.9) | 530 (26.6) | 515 (31.9) | 529 (49.8) | 1681 (49.1) | |||
| = fair (%) | 0 (0.0) | 0 (0.0) | 145 (15.7) | 1629 (30.2) | 250 (12.5) | 236 (14.6) | 256 (24.1) | 1032 (30.1) | |||
| Near Visual Acuity in 2013 | < 0.001 | < 0.001 | |||||||||
| = excellent (%) | 65 (10.1) | 4 (0.4) | 71 (7.7) | 18 (0.3) | 88 (4.4) | 18 (1.1) | 30 (2.8) | 22 (0.6) | |||
| = very good (%) | 246 (38.2) | 53 (4.7) | 311 (33.7) | 181 (3.4) | 336 (16.8) | 101 (6.3) | 167 (15.7) | 187 (5.5) | |||
| = good (%) | 333 (51.7) | 144 (12.7) | 540 (58.6) | 573 (10.6) | 555 (27.8) | 259 (16.0) | 308 (29.0) | 468 (13.7) | |||
| = fair (%) | 0 (0.0) | 679 (59.8) | 0 (0.0) | 2936 (54.4) | 661 (33.1) | 833 (51.6) | 347 (32.6) | 1774 (51.8) | |||
| = poor (%) | 0 (0.0) | 255 (22.5) | 0 (0.0) | 1689 (31.3) | 356 (17.8) | 404 (25.0) | 211 (19.8) | 973 (28.4) | |||
| Near Visual Acuity in 2011 | < 0.001 | < 0.001 | |||||||||
| = excellent (%) | 28 (4.3) | 32 (2.8) | 2 (0.2) | 16 (0.3) | 38 (1.9) | 21 (1.3) | 6 (0.6) | 13 (0.4) | |||
| = very good (%) | 221 (34.3) | 337 (29.7) | 20 (2.2) | 117 (2.2) | 320 (16.0) | 205 (12.7) | 49 (4.6) | 121 (3.5) | |||
| = good (%) | 395 (61.3) | 766 (67.5) | 131 (14.2) | 610 (11.3) | 642 (32.2) | 548 (33.9) | 175 (16.5) | 537 (15.7) | |||
| = fair (%) | 0 (0.0) | 0 (0.0) | 553 (60.0) | 2953 (54.7) | 660 (33.1) | 559 (34.6) | 545 (51.3) | 1742 (50.9) | |||
| = poor (%) | 0 (0.0) | 0 (0.0) | 216 (23.4) | 1701 (31.5) | 336 (16.8) | 282 (17.5) | 288 (27.1) | 1011 (29.5) | |||
| Number of diseases reported in 2013 | 1.00 (1.09) | 1.30 (1.29) | 1.24 (1.29) | 1.60 (1.46) | < 0.001 | 1.12 (1.20) | 1.42 (1.35) | 1.44 (1.42) | 1.71 (1.48) | < 0.001 | |
| Disease Histories in 2013 | |||||||||||
| Heart Diseases (%) | 50 (7.8) | 142 (12.5) | 125 (13.6) | 833 (15.4) | < 0.001 | 191 (9.6) | 212 (13.1) | 148 (13.9) | 599 (17.5) | < 0.001 | |
| Dyslipidemia (%) | 81 (12.6) | 156 (13.7) | 133 (14.4) | 873 (16.2) | 0.023 | 311 (15.6) | 215 (13.3) | 145 (13.6) | 572 (16.7) | 0.006 | |
| Hypertension (%) | 157 (24.4) | 299 (26.3) | 236 (25.6) | 1584 (29.3) | 0.004 | 503 (25.2) | 413 (25.6) | 289 (27.2) | 1071 (31.3) | < 0.001 | |
| Stroke (%) | 17 (2.6) | 35 (3.1) | 29 (3.1) | 231 (4.3) | 0.041 | 58 (2.9) | 57 (3.5) | 23 (2.2) | 174 (5.1) | < 0.001 | |
| Diabetes or high blood sugar (%) | 38 (5.9) | 72 (6.3) | 61 (6.6) | 515 (9.5) | < 0.001 | 140 (7.0) | 122 (7.6) | 82 (7.7) | 342 (10.0) | < 0.001 | |
| Liver disease (%) | 23 (3.6) | 59 (5.2) | 43 (4.7) | 357 (6.6) | 0.002 | 90 (4.5) | 84 (5.2) | 60 (5.6) | 248 (7.2) | < 0.001 | |
| Kidney disease (%) | 31 (4.8) | 84 (7.4) | 59 (6.4) | 541 (10.0) | < 0.001 | 125 (6.3) | 140 (8.7) | 82 (7.7) | 368 (10.7) | < 0.001 | |
| Chronic lung diseases (%) | 46 (7.1) | 116 (10.2) | 90 (9.8) | 675 (12.5) | < 0.001 | 167 (8.4) | 171 (10.6) | 127 (11.9) | 462 (13.5) | < 0.001 | |
| Memory-related disease (%) | 7 (1.1) | 27 (2.4) | 20 (2.2) | 137 (2.5) | 0.143 | 17 (0.9) | 32 (2.0) | 28 (2.6) | 114 (3.3) | < 0.001 | |
| Asthma (%) | 12 (1.9) | 36 (3.2) | 36 (3.9) | 281 (5.2) | < 0.001 | 61 (3.1) | 78 (4.8) | 52 (4.9) | 174 (5.1) | 0.004 | |
| Emotional, nervous or psychiatric problems (%) | 9 (1.4) | 21 (1.9) | 13 (1.4) | 165 (3.1) | 0.001 | 27 (1.4) | 40 (2.5) | 32 (3.0) | 109 (3.2) | < 0.001 | |
| Multimorbidity (%) | 167 (25.9) | 396 (34.9) | 304 (33.0) | 2423 (44.9) | < 0.001 | 588 (29.5) | 626 (38.8) | 422 (39.7) | 1654 (48.3) | < 0.001 | |
Continuous variables were tested to be normally distributed and presented as mean (standard deviation); categorical variables were presented as numerical values (percentages). ANOVA and chi-square tests were used to test the intergroup differences for continuous and categorical variables, respectively
Abbreviations: VI Vision Impairment, NoVI No Vision Impairment, HI Hearing Impairment, NoHI No Hearing Impairment, BMI body mass index
DSI over 2 years also exhibited four patterns: persistent no DSI, transition from DSI to no DSI, transition from no DSI to DSI, and persistent DSI. The differences in demographic characteristics across DSI transition patterns were like those observed in HI transition patterns (Table 2).
Table 2.
Stratified baseline characteristics of participants according to the DSI transition patterns from 2011 to 2013
| NoDSI-NoDSI | NoDSI-DSI | DSI-NoDSI | DSI-DSI | p | |
|---|---|---|---|---|---|
| n | 2377 | 1668 | 1180 | 2873 | |
| Age | 58.81 (8.68) | 59.58 (8.61) | 61.03 (8.81) | 61.63 (8.76) | < 0.001 |
| Sex = male (%) | 1156 (48.6) | 762 (45.7) | 493 (41.8) | 1281 (44.6) | 0.001 |
| BMI (kg/m2) | 24.12 (3.82) | 23.78 (3.77) | 23.98 (3.85) | 23.73 (3.78) | 0.001 |
| Residence Registration Status = agricultural (%) | 1848 (77.7) | 1380 (82.7) | 991 (84.0) | 2410 (83.9) | < 0.001 |
| Marital Status = married (%) | 2091 (88.0) | 1498 (89.8) | 1027 (87.0) | 2524 (87.9) | 0.106 |
| Smoke = yes (%) | 995 (41.9) | 687 (41.2) | 457 (38.7) | 1171 (40.8) | 0.35 |
| Drink | 0.006 | ||||
| = sometimes (%) | 680 (28.6) | 442 (26.5) | 290 (24.6) | 694 (24.2) | |
| = often (%) | 194 (8.2) | 127 (7.6) | 101 (8.6) | 214 (7.4) | |
| = no (%) | 1503 (63.2) | 1099 (65.9) | 789 (66.9) | 1965 (68.4) | |
| Level of Social Activity Engagement | < 0.001 | ||||
| = active (%) | 844 (35.5) | 499 (29.9) | 362 (30.7) | 854 (29.7) | |
| = moderate (%) | 350 (14.7) | 250 (15.0) | 137 (11.6) | 367 (12.8) | |
| = non-participatory (%) | 1183 (49.8) | 919 (55.1) | 681 (57.7) | 1652 (57.5) | |
| Level of Individual Activity Engagement | < 0.001 | ||||
| = active (%) | 261 (11.0) | 128 (7.7) | 110 (9.3) | 192 (6.7) | |
| = moderate (%) | 425 (17.9) | 270 (16.2) | 155 (13.1) | 393 (13.7) | |
| = non-participatory (%) | 1691 (71.1) | 1270 (76.1) | 915 (77.5) | 2288 (79.6) | |
| Physical Fitness Level = low (%) | 467 (19.6) | 379 (22.7) | 327 (27.7) | 764 (26.6) | < 0.001 |
| Grip strength = low (%) | 365 (15.4) | 313 (18.8) | 248 (21.0) | 678 (23.6) | < 0.001 |
| Auditory Acuity in 2013 | < 0.001 | ||||
| = excellent (%) | 131 (5.5) | 0 (0.0) | 51 (4.3) | 0 (0.0) | |
| = very good (%) | 853 (35.9) | 0 (0.0) | 291 (24.7) | 0 (0.0) | |
| = good (%) | 1135 (47.7) | 0 (0.0) | 598 (50.7) | 0 (0.0) | |
| = fair (%) | 221 (9.3) | 1416 (84.9) | 167 (14.2) | 2067 (71.9) | |
| = poor (%) | 37 (1.6) | 252 (15.1) | 73 (6.2) | 806 (28.1) | |
| Auditory Acuity in 2011 | < 0.001 | ||||
| = excellent (%) | 78 (3.3) | 28 (1.7) | 0 (0.0) | 0 (0.0) | |
| = very good (%) | 728 (30.6) | 350 (21.0) | 0 (0.0) | 0 (0.0) | |
| = good (%) | 1368 (57.6) | 1059 (63.5) | 0 (0.0) | 0 (0.0) | |
| = fair (%) | 169 (7.1) | 183 (11.0) | 1008 (85.4) | 2070 (72.1) | |
| = poor (%) | 34 (1.4) | 48 (2.9) | 172 (14.6) | 803 (27.9) | |
| Distance Visual Acuity in 2013 | < 0.001 | ||||
| = excellent (%) | 147 (6.2) | 20 (1.2) | 52 (4.4) | 15 (0.5) | |
| = very good (%) | 569 (23.9) | 77 (4.6) | 253 (21.4) | 92 (3.2) | |
| = good (%) | 790 (33.2) | 177 (10.6) | 386 (32.7) | 185 (6.4) | |
| = fair (%) | 599 (25.2) | 992 (59.5) | 297 (25.2) | 1642 (57.2) | |
| = poor (%) | 272 (11.4) | 402 (24.1) | 192 (16.3) | 939 (32.7) | |
| Distance Visual Acuity in 2011 | < 0.001 | ||||
| = excellent (%) | 76 (3.2) | 29 (1.7) | 7 (0.6) | 4 (0.1) | |
| = very good (%) | 561 (23.6) | 277 (16.6) | 46 (3.9) | 85 (3.0) | |
| = good (%) | 902 (37.9) | 669 (40.1) | 149 (12.6) | 264 (9.2) | |
| = fair (%) | 573 (24.1) | 472 (28.3) | 675 (57.2) | 1535 (53.4) | |
| = poor(%) | 265 (11.1) | 221 (13.2) | 303 (25.7) | 985 (34.3) | |
| Near Visual Acuity in 2013 | < 0.001 | ||||
| = excellent (%) | 114 (4.8) | 4 (0.2) | 35 (3.0) | 5 (0.2) | |
| = very good (%) | 466 (19.6) | 45 (2.7) | 203 (17.2) | 77 (2.7) | |
| = good (%) | 735 (30.9) | 189 (11.3) | 429 (36.4) | 237 (8.2) | |
| = fair (%) | 694 (29.2) | 972 (58.3) | 314 (26.6) | 1635 (56.9) | |
| = poor (%) | 368 (15.5) | 458 (27.5) | 199 (16.9) | 919 (32.0) | |
| Near Visual Acuity in 2011 | < 0.001 | ||||
| = excellent (%) | 51 (2.1) | 18 (1.1) | 4 (0.3) | 5 (0.2) | |
| = very good (%) | 410 (17.2) | 229 (13.7) | 17 (1.4) | 39 (1.4) | |
| = good (%) | 859 (36.1) | 641 (38.4) | 123 (10.4) | 279 (9.7) | |
| = fair (%) | 703 (29.6) | 516 (30.9) | 689 (58.4) | 1598 (55.6) | |
| = poor (%) | 354 (14.9) | 264 (15.8) | 347 (29.4) | 952 (33.1) | |
| Number of diseases reported in 2013 | 1.14 (1.20) | 1.45 (1.36) | 1.47 (1.44) | 1.74 (1.50) | < 0.001 |
| Disease Histories in 2013 | |||||
| Heart Diseases (%) | 238 (10.0) | 229 (13.7) | 176 (14.9) | 507 ( 17.6) | < 0.001 |
| Dyslipidemia (%) | 364 (15.3) | 222 (13.3) | 164 (13.9) | 493 (17.2) | 0.002 |
| Hypertension (%) | 603 (25.4) | 438 (26.3) | 325 (27.5) | 910 (31.7) | < 0.001 |
| Stroke (%) | 70 (2.9) | 58 (3.5) | 33 (2.8) | 151 (5.3) | < 0.001 |
| Diabetes or high blood sugar (%) | 163 (6.9) | 125 (7.5) | 93 (7.9) | 305 (10.6) | < 0.001 |
| Liver disease (%) | 105 (4.4) | 96 (5.8) | 68 (5.8) | 213 (7.4) | < 0.001 |
| Kidney disease (%) | 152 (6.4) | 144 (8.6) | 97 (8.2) | 322 (11.2) | < 0.001 |
| Chronic lung diseases (%) | 201 (8.5) | 188 (11.3) | 151 (12.8) | 387 (13.5) | < 0.001 |
| Memory-related disease (%) | 26 (1.1) | 35 (2.1) | 30 (2.5) | 100 (3.5) | < 0.001 |
| Asthma (%) | 74 (3.1) | 77 (4.6) | 65 (5.5) | 149 (5.2) | 0.001 |
| Emotional, nervous or psychiatric problems (%) | 38 (1.6) | 44 (2.6) | 27 (2.3) | 99 (3.4) | < 0.001 |
| Multimorbidity (%) | 719 (30.2) | 670 (40.2) | 481 (40.8) | 1420 (49.4) | < 0.001 |
Continuous variables were tested to be normally distributed and presented as mean (standard deviation); categorical variables were presented as numerical values (percentages). ANOVA and chi-square tests were used to test the intergroup differences for continuous and categorical variables, respectively
Abbreviations: DSI Dual Sensory Impairment, NoDSI No Dual Sensory Impairment, BMI body mass index
Prospective associations of vision and hearing levels with chronic diseases and multimorbidity vents
Prospective associations of self-reported distance/near vision and hearing levels (excellent, very good, good, fair, poor) with 10 chronic diseases and multimorbidity events
Changes in the proportions of self-reported hearing, distance/near vision levels from 2011 to 2013 and distribution of HI/VI dynamic change patterns are illustrated in Additional File1: Table.S2, Table.S3, Table.S4 and 3D bar charts (Additional File1: Fig.S1).
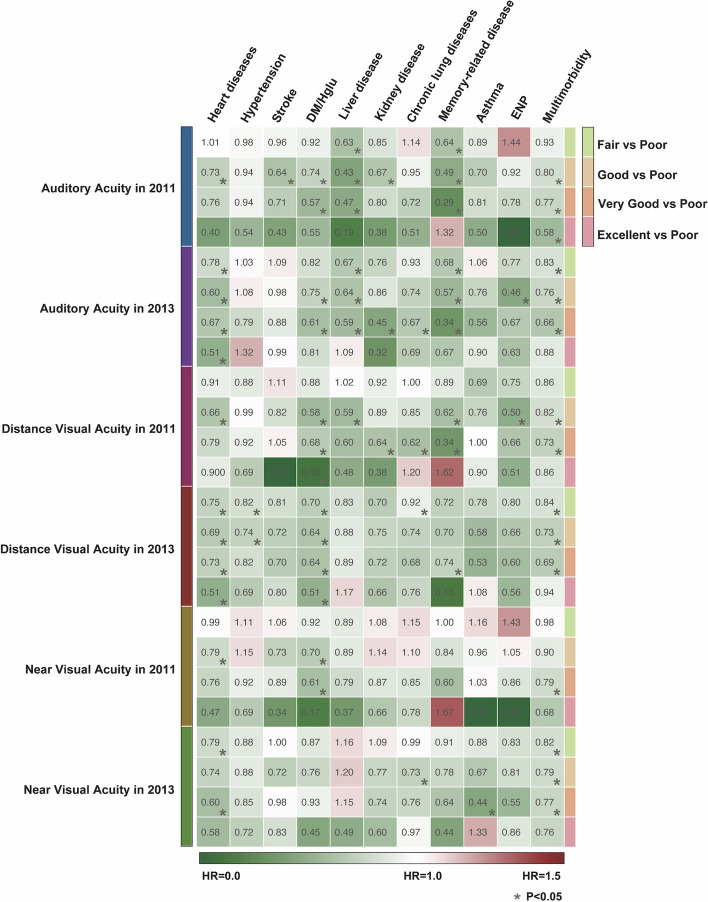
Compared to individuals who reported very poor hearing levels in 2011 or 2013, those who reported fair to excellent hearing levels in the same years exhibited significantly reduced risks (17–72%) of developing new incidents of heart disease, stroke, DM/Hglu, liver disease, kidney disease, chronic lung disease, memory-related diseases, and multimorbidity after 2013 (Fig. 2).
Fig. 2.
Heatmap of the risk effects (aHR) of self-reported levels of auditory and distance/nearvisual acuity on chronic diseases and multimorbidity. aHR adjusted hazard ratios, *, P < 0.05. Abbreviations: DM/Hglu diabetes or high blood sugar, ENP emotional, nervous, or psychiatric problems
Similarly, compared to individuals who reported very poor distance vision levels in 2011 or 2013, those who reported fair to excellent distance vision levels demonstrated significantly lower risks (16–90%) of developing new incidents of heart disease, hypertension, DM/Hglu, liver disease, kidney disease, chronic lung disease, memory-related diseases, ENP, and multimorbidity after 2013 (Fig. 2).
In addition, compared to individuals who reported very poor near vision levels in 2011 or 2013, those who reported fair to excellent near vision levels had significantly lower risks (18–56%) of developing new incidents of heart disease, DM/Hglu, chronic lung disease, asthma, and multimorbidity after 2013 (Fig. 2).
Prospective cumulative incidence of chronic diseases and multimorbidity in VI, HI, and DSI
Kaplan–Meier curves were plotted to calculate the cumulative incidence of 10 chronic diseases and multimorbidity over a 5-year follow-up period for individuals with and without VI, HI, and DSI. The Log-rank test confirmed that, compared to those without HI, individuals with baseline HI had significantly higher cumulative incidence rates of heart disease, chronic lung disease, memory-related diseases, asthma, ENP, and multimorbidity within 5 years. Similarly, compared to those without VI, individuals with baseline VI had significantly higher cumulative incidence rates of heart diseases, DM/Hglu, kidney disease, chronic lung disease, asthma, and multimorbidity within 5 years. Additionally, compared to those without DSI, individuals with baseline DSI had significantly higher cumulative incidence rates of heart diseases, DM/Hglu, kidney disease, chronic lung disease, memory-related disease, asthma, and multimorbidity within 5 years (Additional File1: Fig.S2).
Prospective associations of continuous changes in VI, HI, and DSI with chronic diseases and multimorbidity events
Risk factor contributions of VI, HI, DSI, and their dynamic changes to 10 chronic diseases and multimorbidity
According to the calculated PAF%(95%CIs), baseline HI contributed significantly to the risk of heart diseases (17.42%, 7.58–26.68%), DM/Hglu (12.74%, 1.68–23.14%), chronic lung disease (16.65%, 4.49–27.93%), memory-related disease (25.13%, 6.96–41.00%), asthma (24.22%, 2.12–43.07%), and multimorbidity (9.11%, 2.66–15.39%) to varying degrees. In dynamic measurements, HI reported twice consecutively significantly contributed 10.41–31.04% to the incidence risk of 9 chronic diseases and multimorbidity, excluding hypertension (Additional File1: Table S5, Fig.S3A).
Baseline VI contributed significantly to the risk of heart diseases (16.63%, 0.61–30.52%), kidney disease (26.50%, 3.93–44.50%), chronic lung disease (20.14%, 1.02–36.15%), asthma (42.30%, 8.52–64.66%), and multimorbidity (16.68%, 6.83–25.71%) to varying degrees. In dynamic measurements, persistent VI reported twice consecutively contributed significantly to therisk of DM/Hglu (29.25%, 6.93–47.62%), kidney disease (43.22%, 11.69–65.40%), chronic lung disease (25.89%, 1.00–46.25%), and multimorbidity (22.53%, 10.39–33.66%) (Additional File1:Table S6, Fig.S3B).
Baseline DSI contributed significantly to the incidence risk of heart disease (14.88%, 6.14–23.29%), DM/Hglu (12.92%, 3.19–22.27%), kidney disease (13.69%, 0.87–25.85%), chronic lung disease (18.68%, 8.03–28.77%), memory-related diseases (20.68%, 4.74–35.27%), asthma (28.57%, 9.39–45.33%), and multimorbidity (10.25%, 4.59–15.83%). In dynamic measurements, DSI reported twice consecutively contributed significantly to the incidence risk of 9 chronic diseases and multimorbidity, excluding hypertension (Additional File1: Table S7, Fig.S3C).
Prospective association of VI, HI, and DSI and their dynamic change patterns with the incidence risk of 10 chronic diseases and multimorbidity
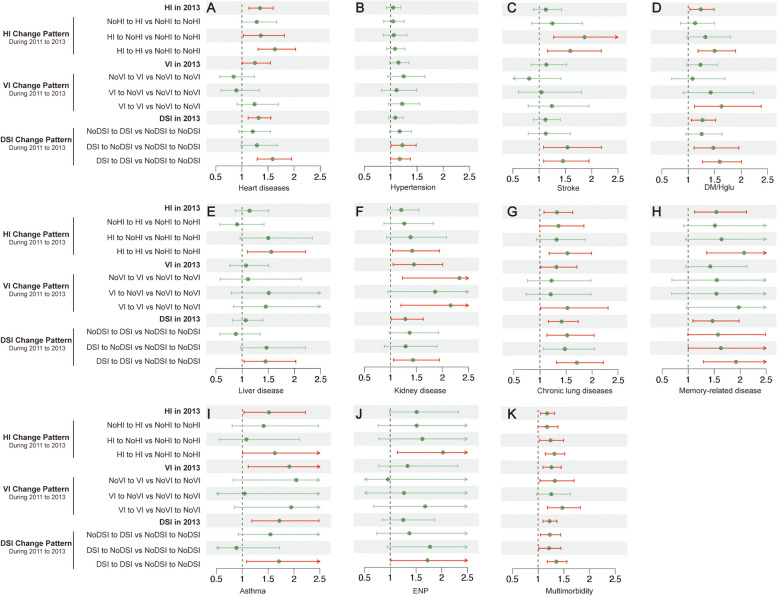
Compared to individuals without HI, those with baseline HI had higher risks of developing heart diseases (P < 0.001, HR 1.35, 95%CI 1.14, 1.60), DM/Hglu (P 0.025, HR 1.24, 95% CI 1.03, 1.49), chronic lung disease (P 0.008, HR 1.33, 95% CI 1.08, 1.63), memory-related diseases (P 0.008, HR 1.54, 95% CI 1.12, 2.12), asthma (P 0.033, HR 1.52, 95% CI 1.04, 2.22), and multimorbidity (P 0.006, HR 1.18, 95% CI 1.05, 1.32). In continuous monitoring, compared to those with persistent no HI, individuals with persistent HI had significantly increased risks of heart diseases (P < 0.001, HR 1.63, 95% CI 1.31, 2.03), stroke (P 0.004, HR 1.59, 95% CI 1.16, 2.18), DM/Hglu (P < 0.001, HR 1.50, 95% CI 1.19, 1.89), liver disease (P 0.013, HR 1.56, 95% CI 1.10, 2.21), kidney disease (P 0.027, HR 1.42, 95% CI 1.04, 1.94), chronic lung disease (P 0.002, HR 1.53, 95% CI 1.17, 1.99), memory-related diseases (P < 0.001, HR 2.08, 95% CI 1.36, 3.18), asthma (P 0.045, HR 1.63, 95% CI 1.01, 2.63), ENP (P 0.016, HR 2.03, 95% CI 1.14, 3.60), and multimorbidity (P < 0.001, HR 1.32, 95% CI 1.14, 1.52) (Table 3, Fig. 3).
Table 3.
Association of HI in 2013 with 10 chronic diseases and multimorbidity new-onset risk and association of HI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort
| HI in 2013 | HI Change Pattern | |||||||
|---|---|---|---|---|---|---|---|---|
| NoHI-HI vs NoHI-HI | HI-NoHI vs NoHI-NoHI | HI-HI vs NoHI-NoHI | ||||||
| aHR (95%CI) | P | aHR (95%CI) | P | aHR (95%CI) | P | aHR (95%CI) | P | |
| Heart diseases | 1.35(1.14–1.60) | < 0.001 | 1.29(0.99–1.67) | 0.060 | 1.36(1.02–1.81) | 0.034 | 1.63(1.31–2.03) | < 0.001 |
| Hypertensiona | 1.05(0.92–1.19) | 0.484 | 1.04 (0.87–1.26) | 0.653 | 1.06(0.86–1.31) | 0.589 | 1.08(0.92–1.27) | 0.332 |
| Stroke | 1.13(0.89–1.43) | 0.312 | 1.25(0.86–1.83) | 0.250 | 1.86(1.27–2.73) | 0.001 | 1.59 (1.16–2.18) | 0.004 |
| DM/Hglub | 1.24(1.03–1.49) | 0.025 | 1.13(0.85–1.50) | 0.403 | 1.32(0.98–1.79) | 0.073 | 1.50(1.19–1.89) | < 0.001 |
| Liver disease | 1.14 (0.87–1.50) | 0.349 | 0.90 (0.57–1.42) | 0.657 | 1.50(0.96–2.34) | 0.076 | 1.56(1.10–2.21) | 0.013 |
| Kidney disease | 1.21(0.95–1.54) | 0.127 | 1.26 (0.88–1.82) | 0.209 | 1.39(0.93–2.07) | 0.111 | 1.42 (1.04–1.94) | 0.027 |
| Chronic lung disease | 1.33(1.08–1.63) | 0.008 | 1.36(1.00–1.84) | 0.054 | 1.32(0.93–1.87) | 0.120 | 1.53(1.17–1.99) | 0.002 |
| Memory-related disease | 1.54 (1.12–2.12) | 0.008 | 1.52(0.91–2.51) | 0.108 | 1.64(0.95–2.84) | 0.076 | 2.08(1.36–3.18) | < 0.001 |
| Asthma | 1.52(1.04–2.22) | 0.033 | 1.41 (0.81–2.47) | 0.225 | 1.08(0.56–2.11) | 0.819 | 1.63 (1.01–2.63) | 0.045 |
| ENP | 1.51 (0.98–2.33) | 0.059 | 1.51 (0.77–2.98) | 0.233 | 1.62(0.78–3.38) | 0.195 | 2.03(1.14–3.60) | 0.016 |
| Multimorbidity | 1.18(1.05–1.32) | 0.006 | 1.17 (1.00–1.39) | 0.057 | 1.24(1.03–1.50) | 0.023 | 1.32(1.14–1.52) | < 0.001 |
All calculated aHRs (95% CI) considered the adjustment for confounding factors including age, sex, BMI, residence registration status, marital status, smoke, drink, level of social and individual activity engagement, physical fitness, grip strength, and medical history of hypertension, dyslipidemia, DM/Hglu
Abbreviations: HI Hearing Impairment, DM/Hglu Diabetes or high blood sugar, ENP Emotional, nervous or psychiatric problems
a In the adjusted analysis of confounding factors, a history of hypertension was exclude
b In the adjusted analysis of confounding factors, a history of DM/Hglu was exclude
Fig. 3.
Forest plots of the risk effects (aHR) of HI/VI/DSI & HI/VI/DSI transition patterns on chronic diseases and multimorbidity. A heart diseases (B) hypertension (C) stroke (D) Diabetes or high blood sugar (E) liver disease (F) kidney disease (G) chronic lung diseases (H) Memory-related disease (I) Asthma (J) Emotional, nervous or psychiatric problems and (K) multimorbidity
Compared to individuals without VI, those with early VI exhibited higher risks of developing heart diseases (P 0.042, HR 1.25, 95% CI 1.01, 1.55), kidney disease (P 0.024, HR 1.45, 95% CI 1.05, 2.00), chronic lung disease (P 0.040, HR 1.32, 95% CI 1.01,1.71), asthma (P 0.018, HR 1.91, 95% CI 1.12, 3.28), and multimorbidity (P 0.001, HR 1.26, 95% CI 1.10,1.45). Continuous monitoring revealed that persistent VI was significantly associated with increased risks of DM/Hglu (P:0.012,HR:1.63, 95% CI 1.11, 2.38), kidney disease (P 0.010, HR 2.16, 95% CI 1.20, 3.87), chronic lung disease (P 0.042, HR 1.53, 95% CI 1.02, 2.31), and multimorbidity (P < 0.001, HR 1.47, 95% CI 1.19, 1.82), with higher effect sizes compared to single time reported VI (Table 4, Fig. 3).
Table 4.
Association of VI in 2013 with 10 chronic diseases and multimorbidity new-onset risk and association of VI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort
| VI in 2013 | VI Change Pattern | |||||||
|---|---|---|---|---|---|---|---|---|
| NoVI-VI vs NoVI-VI | VI-NoVI vs NoVI-NoVI | VI-VI vs NoVI-NoVI | ||||||
| aHR(95%CI) | P | aHR(95%CI) | P | aHR(95%CI) | P | aHR(95%CI) | P | |
| Heart diseases | 1.25(1.01–1.55) | 0.042 | 0.84(0.57–1.24) | 0.388 | 0.90(0.60–1.33) | 0.584 | 1.24(0.91–1.70) | 0.172 |
| Hypertensiona | 1.15(0.98–1.35) | 0.094 | 1.25 (0.94–1.65) | 0.125 | 1.11(0.83–1.50) | 0.484 | 1.22(0.95–1.55) | 0.116 |
| Stroke | 1.14(0.85–1.52 | 0.388 | 0.81(0.46–1.42) | 0.459 | 1.05(0.61–1.80) | 0.873 | 1.24 (0.79–1.95) | 0.342 |
| DM/Hglub | 1.23(0.97–1.56) | 0.086 | 1.08(0.69–1.70) | 0.743 | 1.42(0.91–2.23) | 0.126 | 1.63(1.11–2.38) | 0.012 |
| Liver disease | 1.07 (0.77–1.51) | 0.677 | 1.11 (0.58–2.13) | 0.753 | 1.51(0.79–2.87) | 0.210 | 1.45 (0.84–2.52) | 0.185 |
| Kidney disease | 1.45 (1.05–2.00) | 0.024 | 2.33(1.23–4.39) | 0.009 | 1.86(0.96–3.62) | 0.068 | 2.16(1.20–3.87) | 0.010 |
| Chronic lung disease | 1.32(1.01–1.71) | 0.040 | 1.22 (0.76–1.98) | 0.411 | 1.21(0.74–1.98) | 0.458 | 1.53 (1.02–2.31) | 0.042 |
| Memory-related disease | 1.42(0.95–2.13) | 0.085 | 1.55 (0.69–3.49) | 0.288 | 1.54 (0.68–3.51) | 0.300 | 1.97(0.97–4.03) | 0.062 |
| Asthma | 1.91 (1.12–3.28) | 0.018 | 2.05(0.82–5.08) | 0.123 | 1.04 (0.37–2.94) | 0.936 | 1.94(0.85–4.45) | 0.116 |
| ENP | 1.34 (0.77–2.31) | 0.299 | 0.96 (0.32–2.85) | 0.934 | 1.27(0.43–3.72) | 0.664 | 1.68(0.68–4.16) | 0.263 |
| Multimorbidity | 1.26(1.10–1.45) | 0.001 | 1.33(1.04–1.70) | 0.025 | 1.26(0.97–1.63) | 0.081 | 1.47 (1.19–1.82) | < 0.001 |
All calculated aHRs (95% CI) considered the adjustment for confounding factors including age, sex, BMI, residence registration status, marital status, smoke, drink, level of social and individual activity engagement, physical fitness, grip strength, and medical history of hypertension, dyslipidemia, DM/Hglu
Abbreviations: VI Vision Impairment, DM/Hglu Diabetes or high blood sugar, ENP Emotional, nervous or psychiatric problems
a In the adjusted analysis of confounding factors, a history of hypertension was exclude
b In the adjusted analysis of confounding factors, a history of DM/Hglu was exclude
Compared to individuals without DSI, those with early DSI were closely associated with higher risks of heart diseases (P < 0.001, HR 1.32, 95% CI 1.12, 1.55), DM/Hglu (P 0.010, HR 1.27, 95% CI 1.06, 1.52), kidney disease (P 0.037, HR 1.29, 95% CI 1.02, 1.63), chronic lung disease (P < 0.001, HR 1.42, 95% CI 1.16, 1.73), memory-related disease (P 0.012, HR 1.47, 95% CI 1.09, 1.98), asthma (P 0.004, HR 1.72, 95% CI 1.19, 2.49), and multimorbidity (P < 0.001, HR 1.22, 95% CI 1.09, 1.37). In continuous tracking, individuals with persistent DSI had higher risks of developing heart diseases (P < 0.001, HR 1.59, 95% CI 1.29, 1.95), stroke (P 0.012, HR 1.45, 95% CI 1.08, 1.95), DM/Hglu (P < 0.001, HR 1.60, 95% CI 1.27, 2.01), liver disease (P 0.030, HR 1.45, 95% CI 1.04, 2.02), kidney disease (P 0.019, HR 1.44, 95% CI 1.06–1.94), chronic lung disease (P < 0.001, HR 1.71, 95% CI 1.32, 2.21), memory-related diseases (P 0.001, HR 1.92, 95% CI 1.29,2.85), asthma (P 0.020, HR 1.71, 95% CI 1.09, 2.69), ENP (P 0.045, HR 1.72, 95% CI 1.01, 2.93), and multimorbidity (P < 0.001, HR 1.36, 95% CI 1.18, 1.56) compared to those with persistent no DSI (Table 5, Fig. 3).
Table 5.
Association of DSI in 2013 with 10 chronic diseases and multimorbidity new-onset risk and association of DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort
| DSI in 2013 | DSI Change Pattern | |||||||
|---|---|---|---|---|---|---|---|---|
| NoDSI-DSI vs NoDSI-DSI | DSI-NoDSI vs NoDSI-NoDSI | DSI-DSI vs NoDSI-NoDSI | ||||||
| aHR(95%CI) | P | aHR(95%CI) | P | aHR(95%CI) | P | aHR(95%CI) | P | |
| Heart diseases | 1.32(1.12–1.55) | < 0.001 | 1.21(0.95–1.55) | 0.130 | 1.29(0.99–1.68) | 0.061 | 1.59(1.29–1.95) | < 0.001 |
| Hypertensiona | 1.09(0.96–1.24) | 0.180 | 1.17(0.98–1.40) | 0.084 | 1.22(1.01–1.49) | 0.044 | 1.17(1.00–1.38) | 0.052 |
| Stroke | 1.12(0.89–1.40) | 0.324 | 1.13(0.79–1.60) | 0.510 | 1.54(1.09–2.19) | 0.016 | 1.45(1.08–1.95) | 0.012 |
| DM/Hglub | 1.27(1.06–1.52) | 0.010 | 1.25(0.96–1.64) | 0.102 | 1.47(1.11–1.96) | 0.007 | 1.60(1.27–2.01) | < 0.001 |
| Liver disease | 1.07(0.82–1.39) | 0.632 | 0.88(0.57–1.34) | 0.548 | 1.47(0.97–2.21) | 0.068 | 1.45(1.04–2.02) | 0.030 |
| Kidney disease | 1.29(1.02–1.63) | 0.037 | 1.37(0.98–1.93) | 0.069 | 1.29(0.88–1.89) | 0.189 | 1.44(1.06–1.94) | 0.019 |
| Chronic lung disease | 1.42(1.16–1.73) | < 0.001 | 1.52(1.14–2.04) | 0.005 | 1.48(1.07–2.05) | 0.019 | 1.71(1.32–2.21) | < 0.001 |
| Memory-related disease | 1.47(1.09–1.98) | 0.012 | 1.57(1.00–2.49) | 0.052 | 1.63(1.00–2.65) | 0.049 | 1.92(1.29–2.85) | 0.001 |
| Asthma | 1.72(1.19–2.49) | 0.004 | 1.55(0.93–2.58) | 0.097 | 0.89(0.46–1.72) | 0.726 | 1.71(1.09–2.69) | 0.020 |
| ENP | 1.25(0.84–1.87) | 0.266 | 1.37(0.74–2.56) | 0.319 | 1.78(0.94–3.34) | 0.076 | 1.72(1.01–2.93) | 0.045 |
| Multimorbidity | 1.22(1.09–1.37) | < 0.001 | 1.23(1.05–1.44) | 0.011 | 1.21(1.02–1.45) | 0.032 | 1.36(1.18–1.56) | < 0.001 |
All calculated aHRs (95% CI) considered the adjustment for confounding factors including age, sex, BMI, residence registration status, marital status, smoke, drink, level of social and individual activity engagement, physical fitness, grip strength, and medical history of hypertension, dyslipidemia, DM/Hglu
Abbreviations: DSI Dual Sensory Impairment, DM/Hglu Diabetes or high blood sugar, ENP Emotional, nervous or psychiatric problems
a In the adjusted analysis of confounding factors, a history of hypertension was exclude
b In the adjusted analysis of confounding factors, a history of DM/Hglu was exclude
Sensitivity analysis
The primary results regarding the risk effects of HI, VI, DSI, and their transition patterns on the incidence of new chronic diseases and multimorbidity were robust in the sensitivity analyses.
First, analysis with imputed age and BMI data yielded results similar to the original analysis (Additional File1: Table.S8, Table.S9, Table.S10, Fig.S4).
Then, reanalysis of the dataset after multianalysis again produced similar results (Additional File1: Table.S11, Table.S12, Table.S13, Fig.S5). This indicates that the risk associated with HI, VI, DSI, or their transition patterns was not biased by differences in baseline characteristics such as age, gender, BMI, household registration and marital status, smoking and drinking status, social and personal activity levels, low physical fitness and low grip strength, history of hypertension, hyperlipidemia, and DM/Hglu.
Next, after excluding individuals who developed the disease within the first year of follow-up, reanalysis highlighted a significant association between dynamic patterns of VI and increased risk of chronic kidney disease, compared to those with persistent no VI. However, the associations with heart disease, chronic lung disease, and asthma did not reach statistical significance. For HI and DSI and their dynamic patterns, the primary results remained consistent in the reanalysis. These findings provide further support for the causal relationship between early HI, VI, and DSI and the subsequent development of chronic diseases and multimorbidity (Additional File1: Table.S14, Table.S15, Table.S16, Fig.S6).
Finally, we then examined the effect of sensory aids. In 2013, hearing aid use was rare (0.4%), while 7.45% regularly used visual aids and 21.47% used them occasionally. Including sensory aid use as model covariates yielded results similar to our primary findings, indicating that aid use did not modify the relationship between sensory impairment and chronic disease risk (Additional File1: Table.S17, Table.S18, Table.S19, Fig.S7).
VI, HI, and chronic diseases: results from MR analysis
A total of 27 genetic instruments significantly impacting HI were identified in our MR analysis (Additional File1: Table.S20). We noted HI was strongly associated with a higher risk of four outcomes (P < 0.00104): VHD [OR_IVW, 95%CI 2.70, 1.51–4.85], hypertension [OR_IVW, 95%CI 1.94, 1.39–2.72)], renal failure [OR_IVW, 95%CI 4.65, 2.24–9.68], and schizophrenia (SCZ) [OR_IVW, 95%CI 2.12, 1.37–3.28]. Additionally, HI was suggestively associated with a higher risk of six outcomes (0.00104 < P < 0.05): AF [OR_IVW, 95%CI 2.23, 1.28–3.91)], CHD [OR_IVW,95%CI 1.87, 1.09–3.20)], HF [OR_IVW, 95%CI 2.06, 1.30–3.28)], stroke [OR_IVW, 95%CI 1.61, 1.01–2.55], chronic kidney disease (CKD) [OR_IVW, 95%CI 2.00, 1.17–3.42)], and bronchitis [OR_IVW, 95%CI 2.60, 1.05–6.41] (Fig. 4A and Additional File1:Table.S21). Moreover, the corresponding 99.896%CI calculated based on the Bonferroni-corrected α_adjusted is displayed in Additional File1: Fig.S8 A.
Fig. 4.
Forest plots of the risk effects (OR, 95%CI) of genetically predicted VI/HI on chronic diseases. A The causal relationship between genetically predicted VI and 24 outcomes by the IVW method. B The causal relationships between HI and the risk of 24 outcomes by the IVW method. The yellow repentant suggestive associations were noted between exposure and outcomes(0.00104<P<0.05), while red repentant strong associations were noted between exposure and outcomes(P<0.00104). 95% CI is presented in the forest plot, with a supplementary forest plot showing wider 99.896% CI calculated using Bonferroni-corrected α_adjusted. Abbreviation: SNP, single nucleotide polymorphism; OR:odds ratio, CI:confidence interval; IVW: inverse‐variance weighted; VI:visual impairment; HI:hearing impairment; AF: Atrial fibrillation and flutter, VHD: valvular heart disease, CHD: Major coronary heart disease event, HF: heart failure, GLU: Blood glucose levels, T2D:type 2 diabetes, F&CL: Fibrosis and cirrhosis of liver, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, PHD: Pulmonary heart disease, FVC:Forced vital capacity, FEV1:Forced expiratory volume in 1-second, PD:Parkinson's disease, AD:alzheimer's disease, MDD:Major depressive disorder, SCZ: schizophrenia
After selection, 48 independent SNPs significantly associated with VI were used in our MR analysis (Additional File1: Table.S22). The IVW model revealed suggestive associations between VI and an elevated risk of four conditions (0.00104 < P < 0.05): hepatic failure [OR_IVW, 95%CI 1.15, 1.01–1.31)], reduced lung function (FEV1/FVC) [OR_IVW, 95%CI 0.98, (0.97, 0.99)], Parkinson’s disease (PD) [OR_IVW, 95%CI 1.07, 1.04–1.11], and Alzheimer’s disease (AD) [OR_IVW, 95%CI 1.12, 1.02–1.24]. Furthermore, strong associations were identified between VI and an increased risk of threeconditions (P < 0.00104): Blood glucose levels (GLU) [OR_IVW, 95%CI 1.02,1.01 − 1.03)], T2D [OR_IVW, 95%CI 1.06, 1.04–1.08], and reduced lung function (FVC) [OR_IVW, 95%CI 0.99, 0.98–0.99] (Fig. 4B and Additional File1:Table.S21). Moreover, the corresponding 99.896%CI calculated based on the Bonferroni-corrected α_adjusted is displayed in Additional File1: Fig.S8 B.
Using the weighted median and MR Egger regression methods, consistent directionality of effect estimates was noted in all significant associations (Additional File1: Table.S21), thereby confirming the outcomes of the IVW model.
As previously stated, in instances where outliers were identified, they were excluded, and the causal estimation was reassessed. As a result, no evidence of pleiotropy was detected in any of the analyses. No heterogeneity was detected in the Cochran’s Q analysis (P > 0.05) (Additional File1: Table.S21). Furthermore, the “leave-one-out” analysis revealed that the exclusion of each SNP did not lead to any significant changes in the outcomes.
Scatter plots and “leave-one-out” tests of VI/HI with outcomes of 24 chronic diseases were presented in supplementary Additional File1: Fig.S9- Fig.S56.
Discussion
This study involved repeated measurements of hearing, vision, and dual-sensory levels within a prospective cohort study (CS), analyzing the patterns of change in relation to the risk of various chronic diseases and multimorbidity. We found that dynamic changes in HI/VI/DSI status significantly increased the risk of incident 10 chronic diseases and multimorbidity. Mendelian randomization confirmed significant or potential genetic associations between HI/VI and 8 chronic diseases, including heart diseases, hypertension, stroke, DM/Hglu, kidney disease, lung disease, memory disease, and ENP (Fig. 5).
Fig. 5.
Key findings from the association analysis of sensory impairments and disease risks: a summary of positive results from cohort studies and mendelian randomization, along with potential pathophysiological mechanisms. A checkmark indicates positive results in the respective studies. In Mendelian randomization analyses, both strong and suggestive associations are encompassed. Abbreviations: HI Hearing Impairment, VI Vision Impairment, DSI Dual Sensory Impairment, ENP Emotional, nervous or psychiatric problems
From a mechanism perspective, the association between HI/VI/DSI and various chronic diseases has both commonalities and specificities. Common pathways that may link auditory and visual impairments to disease onset include chronic systemic inflammation, vascular dysfunction, immune activation, and shared health and metabolic risk factors. However, some specific pathogenic mechanisms of the diseases themselves and the risk gene loci shared with auditory and visual impairments differ and require special analysis.
AD and PD are common neurodegenerative disorders affecting memory function in middle-aged and elderly populations. This association may be linked through the extensive involvement of the dopaminergic neuronal system in the regulation of auditory [28] and retinal functions [29, 30]. Dopaminergic amacrine cells in the retina play a crucial role in visual modulation, such as light response [31]. The accumulation of β-amyloid [32] and tau proteins [33] in both the brain and the retina/optic nerve simultaneously affects memory function and sensory levels [34]. Neurodegenerative changes in regions such as the basal ganglia, hippocampus, temporal lobe auditory cortex, and occipital lobe visual cortex connect memory decline with the clinical manifestations of sensory impairments [35]. Additionally, oxidative stress [36], mitochondrial dysfunction [37], and neuroinflammation [38] in brain regions like the substantia nigra, cortical areas, microglia, and sensory neurons contribute to the development of PD/AD and associated sensory impairments.
Emotional, nervous or psychiatric problems (ENP) are often comorbid with sensory impairments, particularly visual and auditory deficits [39]. The link between hearing loss and psychiatric disorders may be explained by shared genetic factors and neuroimmune responses. Overlapping single-nucleotide polymorphisms between hearing loss [40] and brain disorders such as schizophrenia and psychosis [41] suggest a potential genetic basis for this connection. Furthermore, from a neuroinflammatory and immune response perspective [42], during aging, macrophages in the cochlea and microglia in the auditory brainstem exhibit activation patterns consistent with chronic inflammation in their microenvironment [43]. This immune activation releases tissue-damaging factors, leading to damage in the auditory nerve and cochlear structures, thereby contributing to HI [44]. Concurrently, in this inflammatory context, there is overactivation of dopamine D3 receptors and interferon-γ synthesis in lymphocytes. The interaction between these pathways and the glutamate system may trigger key mechanisms in the pathogenesis of schizophrenia [45].
The impact of early exposure to HI/VI on cardiovascular diseases currently lacks a definitive consensus. Mechanistically, proper inner ear microcirculation is crucial for maintaining the function of the stria vascularis cells, including intermediate cells, basal cells, and marginal cells [46]. Stria vascularis dysfunction within the cochlea [47] is a key mechanism underlying sensorineural hearing loss [48] and shares pathogenic pathways with cardiovascular diseases, such as atherosclerosis, inflammation [49], neurohormonal dysfunction [50], vasospasm [51], and micro emboli formation [52]. These conditions also share common risk factors like hypertension, diabetes, and dyslipidemia [53]. The microvascular system of the inner ear is particularly fragile, and any degeneration or microcirculatory disturbance in the stria vascularis can quickly manifest as a decline in hearing [53]. In contrast, the compensatory systems of overall cardiac function and cerebral blood supply are more robust, and further systemic vascular deterioration may be necessary before progressing to heart diseases and stroke.
Similarly, vision loss is a well-established complication of diabetes, yet there has been little focus on whether early VI could serve as a risk factor for new-onset diabetes. From the perspective of psychological stress and physiological responses, VI can trigger psychological stress, which activates the hypothalamic–pituitary–adrenal axis, leading to excessive cortisol secretion [54, 55]. This, in turn, contributes to insulin resistance and increased fat accumulation, thereby raising the risk of DM/Hglu [56, 57]. From the anachronic inflammation perspective, both the stress state induced by aging and VI contribute to a chronic systemic inflammatory condition and accelerated insulin resistance [58], thereby increasing diabetes risk [59]. From a metabolic perspective, reduced physical activity in individuals with VI can lead to weight gain, further elevating the risk of DM/Hglu. Consequently, by the time metabolic abnormalities have progressed to DM, vision decline may have already been present for a considerable period.
The association between sensory impairments and chronic lung disease is rarely addressed in clinical practice. Chronic, systemic inflammation in the body is a shared risk factor linking sensory impairments [60] and chronic lung diseases, which may explain their association. Inflammation can promote retinal inflammation and vascular remodeling through exosomes and macrovesicles [61], directly affect the optic nerve leading to optic neuritis [62] and demyelination and reduce optic nerve blood supply through vascular inflammation. Additionally, systemic inflammation can lead to subretinal yellow deposits, changes in retinal pigment epithelium [63], and choroidal neovascularization, contributing to macular degeneration. Similarly, immunosenescence and chronic inflammation can lead to ischemia and fluid imbalance in the inner ear by affecting its microcirculation [64]. Furthermore, chronic inflammation and oxidative stress can promote the apoptosis of lung epithelial and vascular endothelial cells through direct induction or excessive autophagy [65]. Macrophages and neutrophils release matrix metalloproteinases, leading to alveolar structure destruction and airway remodeling, while airway smooth muscle cell proliferation and connective tissue deposition result in airway narrowing and fibrosis [66, 67], contributing to the development of chronic lung diseases. When the sensitivity of the optic nerve, retinal vessels, auditory nerves, and hair cells to systemic inflammation is higher than that of pulmonary vessels and airway smooth muscle cells, sensory impairments may precede the onset of chronic lung diseases.
The comorbidity between kidney disease and hearing loss is notably prevalent. The complex interrelationship between these conditions may be explained by several factors: both the kidneys and auditory organs share a common embryonic origin [68] as well as gene signaling networks [69]. Furthermore, at the cellular level, the auditory organs and kidneys depend on similar biological structures and functional mechanisms [70–72]. Similar to cardiovascular diseases, the functionality of the kidneys and cochlea is also associated with good vascular function and adequate microcirculation. Therefore, vascular aging, oxidative stress, and chronic inflammation can promote the decline in both hearing and kidney function [73]. Given the genetic, structural, and functional connections between the auditory organs and kidneys, a loss in auditory function in response to pathological stimuli may serve as an early indicator of impending adverse outcomes in renal tissue.
The association between multimorbidity and sensory impairments is closely intertwined. Several potential mechanisms can explain this association: Social and Psychological Impact: Sensory impairments directly affect psychological well-being and social functioning. Increased feelings of depression and loneliness can lead to varying degrees of social isolation [74–76], reduced physical activity [77], and heightened stress, which may be pathways to an increased risk of chronic diseases. Vascular Aging: The decline in vascular function is often first reflected in auditory and visual functions. Both the inner ear and retina rely on fine microvascular blood supply to maintain their physiological functions [78–81]. Early hearing and vision loss may be external manifestations of stria vascularis dysfunction in the cochlea, which can then affect the function of various target organs and develop into a range of chronic conditions. Systemic Inflammation: In the auditory system, inflammation can lead to structural damage in the inner ear, including hair cells and auditory nerves [82]. Cytokines and oxidative stress factors activate macrophages and microglia, resulting in hair cell death [83]. Similarly, in visual systems, inflammatory mediators promote oxidative stress and angiogenesis in the retina, leading to vision loss [84]. Additionally, inflammation can activate the hypothalamic–pituitary–adrenal axis, contributing to metabolic disorders and insulin resistance [85], thereby increasing the risk of metabolic and cardiovascular diseases [49], cognitive decline [86], and, ultimately, the risk of multimorbidity.
This study has several limitations. First, the assessment of vision and hearing levels relied on self-evaluation, which may introduce bias, and the reporting of chronic diseases was also self-reported, possibly leading to underestimation of prevalence and reporting delays. The lack of detailed information on chronic disease subtypes requires validation in more specific disease cohorts.
Additionally, the observational study population primarily consists of East Asians, whereas the MR study data mainly originate from European populations. Due to the current lack of GWAS data on exposures or outcomes from East Asian populations, it is not possible to analyze the genetic associations between sensory impairments and disease risks specific to East Asians. Furthermore, since there is also a lack of GWAS data related to DSI, genetic association analysis on the risk of diseases related to DSI remains challenging, and this area of genetic risk evaluation is still unexplored. Therefore, caution is required when integrating conclusions from cohort studies and genetic association analyses. Future investigations should aim to conduct observational studies on European populations, genetic analyses on East Asians, and track the release of GWAS data on DSI to address the limitations of this study.
Conclusions
In summary, this study systematically explored the prospective incidence risk of 10 common chronic diseases and multimorbidity with early exposure and continuous changes in HI, VI, and DSI. Combining MR studies, it confirmed causal associations at the genetic level. The findings underscore the importance of early and continuous monitoring of sensory levels in preventing chronic disease onset, highlighting the potential of identifying and managing sensory function decline to reduce chronic disease risk and serving as a healthcare strategy for high-risk populations.
Supplementary Information
Additional file 1. Part 1 includes introduction to The China Health and Retirement Longitudinal Study (CHARLS) and basic characters evaluation methods. Part 2 includes MR methods. Table. S1 Data sources for exposures and outcomes. Part 3 includes 22 tables, and 56 figures related to the results of cohort studies and Mendelian randomization studies. Table. S2-S4 Number and proportion of individuals at five different levels of auditory acuity/ distance visual acuity/ near visual acuity in 2011 and 2013. Table. S5-S7 Estimated burden of chronic diseases and multimorbidity attributable to HI/VI/DSI and HI/VI/DSI transition patterns. Table.S8-S10 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort using multiple imputation approach. Table.S11-S13 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort using the propensity scores of overlap weighting. Table.S14-S16 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk after excluding those with outcomes occurring within the first year in the CHARLS cohort. Table.S17-S19 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk after adjusted for the use of hearing/visual aids. Table.S20 Genetic variants that were used as instruments for VI. Table.S21 MR analysis with random-effects IVW, MR-Egger, weighted median for association between VI/HI and 17 outcomes, MR-Egger intercept/MRPRESSO test for horizontal pleiotropy, and heterogeneity tests with Cochran’s Q statistic. Table.S22 Genetic variants that were used as instruments for HI. Fig. S1 Distribution of Hearing and Vision Levels Reported in 2011 and 2013 (A), Distribution of Hearing Levels B) Population Distribution of HI Dynamic Change Patterns C) Distribution of Distance Vision Levels D) Distribution of Near Vision Levels E) Population Distribution of VI Dynamic Change Patterns. Fig. S2 Kaplan-Meier plots of the cumulative incidence to new-onset chronic diseases and multimorbidity in sub-cohorts, stratified by whether HI/VI/DSI in 2013. Fig. S3 PAF%(95%CI) for chronic diseases and multimorbidity due to HI/VI/DSI & HI/VI/DSI transition patterns. Fig.S4-S7 Sensitivity analysis heatmap: impact of data imputation, PS matching, first-year event exclusion, and sensory aid adjustment. Fig. S8 Forest plots of the risk effects (OR, 99.896%CI) of genetically predicted VI/HI on chronic diseases. Fig.S9-S56 Scatter plot for the causal association between HI/VI and 24 disease status and leave-one-out tests.
Abbreviations
- AD
Alzheimer’s disease
- AF
Atrial fibrillation and flutter
- CHARLS
The China Health and Retirement Longitudinal Study
- CHD
Major coronary heart disease event
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CS
Cohort study
- DIAMANTE
The Diabetes Meta-Analysis of Trans-Ethnic association studies
- DM/glu
Diabetes or high blood sugar
- DSI
Dual sensory impairments
- ENP
Emotional, nervous or psychiatric problems
- F&CL
Fibrosis and cirrhosis of liver
- FEV1
Forced expiratory volume in 1-s
- FVC
Forced vital capacity
- GLU
Blood glucose levels
- GWAS
Genome-wide association studies
- HF
Heart failure
- HI
Hearing impairments
- IGAP
International Genomics of Alzheimer’s Project
- MDD
Major depressive disorder
- MR
Mendelian randomization analysis
- MRC-IEU
The Medical Research Council-Integrative Epidemiology Unit
- PD
Parkinson’s disease
- PGC
The Psychiatric Genomics Consortium
- PHD
Pulmonary heart disease
- SCZ
Schizophrenia
- T2D
Type 2 diabetes
- VHD
Valvular heart disease
- VI
Vision impairments
Authors' contributions
YW, JW, and WL conceived the research topic and framework. YW, NH, and YH conducted data cleaning and analysis for the cohort study. FC and SZ completed the Mendelian randomization analysis. YW, FC, and YT drafted the manuscript. YW, WG, and JP reviewed the data and revised the manuscript. JW secured funding for the project. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Key R&D Program of China (Nos. 2018YFC2002100 and 2018YFC2002103) and the Key R&D Support Program of Chengdu (Nos.2022YF0900001SN) to JW.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
CHARLS has received ethical approval from the Institutional Review Board of Peking University (Approved number: IRB00001052-11015), and all participants provided written informed consent.
Consent for publication
All the listed authors have seen and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaoling Wang, Fang Cheng, Niuniu Hou and Yuting Tan are the co-first authors of this paper.
Contributor Information
Wei Li, Email: drwli@hust.edu.cn.
Jinhui Wu, Email: wujinhui@scu.edu.cn.
References
- 1.GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996–1009. 10.1016/S0140-6736(21)00516-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bright T, Ramke J, Zhang JH, et al. Prevalence and impact of combined vision and hearing (dual sensory) impairment: a scoping review. PLOS Glob Public Health. 2023;3(5):e0001905. 10.1371/journal.pgph.0001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study [published correction appears in Lancet Glob Health. 2021 Apr;9(4):e408. 10.1016/S2214-109X(21)00050-4]. Lancet Glob Health. 2021;9(2):e144-e160. 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed]
- 4.Looi LM, Ganten D, McGrath PF, Gross M, Griffin GE. Hearing loss: a global health issue. Lancet. 2015;385(9972):943–4. 10.1016/S0140-6736(15)60208-2. [DOI] [PubMed] [Google Scholar]
- 5.Pula JH, Yuen CA. Eyes and stroke: the visual aspects of cerebrovascular disease. Stroke Vasc Neurol. 2017;2(4):210–20. 10.1136/svn-2017-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Liao H, Wang W, Scheetz J, Zhang J, He M. Visual impairment and major eye diseases in chronic kidney disease: the National Health and Nutrition Examination Survey, 2005–2008. Am J Ophthalmol. 2020;213:24–33. 10.1016/j.ajo.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee H. Recent advances in acute hearing loss due to posterior circulation ischemic stroke. J Neurol Sci. 2014;338(1–2):23–9. 10.1016/j.jns.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Mamo SK, Pearlman J, Wheeler KA. Associations between age-related hearing loss, cognitive impairment, and multiple chronic conditions in a Group Care Setting. J Speech Lang Hear Res JSLHR. 2023;66(12):5087–108. 10.1044/2023_JSLHR-23-00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samocha-Bonet D, Wu B, Ryugo DK. Diabetes mellitus and hearing loss: a review. Ageing Res Rev. 2021;71:101423. 10.1016/j.arr.2021.101423. [DOI] [PubMed] [Google Scholar]
- 10.Wells HRR, Freidin MB, Zainul Abidin FN, et al. GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK biobank. Am J Hum Genet. 2019;105(4):788–802. 10.1016/j.ajhg.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue Z, Yuan J, Chen F, et al. Genome-wide association meta-analysis of 88,250 individuals highlights pleiotropic mechanisms of five ocular diseases in UK Biobank. EBioMedicine. 2022;82:104161. 10.1016/j.ebiom.2022.104161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18. https://r10.finngen.fi/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welter D, MacArthur J, Morales J, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001-1006 GWAS Catalog. https://www.ebi.ac.uk/gwas/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan A, Spracklen CN, Zhang W, et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet. 2022;54:560–72. 10.1038/s41588-022-01058-3. [DOI] [PMC free article] [PubMed]
- 15.Wuttke M, Li Y, Li M, et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet. 2019;51:957–72. 10.1038/s41588-019-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taliun D, Harris DN, Kessler MD, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590:290–9. 10.1038/s41586-021-03205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsworth B, Lyon M, Alexander T, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv, 2020: 2020.08.10.244293. IEU Open GWAS Project https://gwas.mrcieu.ac.uk/datasets/ieu-b-7/.
- 18.Trubetskoy V, Pardiñas AF, Qi T, et al; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. https://figshare.com/articles/dataset/MDD2_MDD2018_GWAS_sumstats_w_o_UKBB/21655784. [DOI] [PMC free article] [PubMed]
- 19.Trubetskoy V, Pardiñas AF, Qi T, et al.; Schizophrenia Working Group of the Psychiatric Genomics Consortium. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604(7906):502-508. https://figshare.com/articles/dataset/scz2022/19426775. [DOI] [PMC free article] [PubMed]
- 20.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313–29. 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtin F, Schulz P. Multiple correlations and Bonferroni’s correction. Biol Psychiatry. 1998;44(8):775–7. 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 24.Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40. 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 25.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiol Camb Mass. 2017;28(1):30–42. 10.1097/EDE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen APF, Malgady JM, Chen L, et al. Nigrostriatal dopamine pathway regulates auditory discrimination behavior. Nat Commun. 2022;13(1):5942. 10.1038/s41467-022-33747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinane C, Calligaro H, Jandot A, et al. Dopamine modulates the retinal clock through melanopsin-dependent regulation of cholinergic waves during development. BMC Biol. 2023;21(1):146. 10.1186/s12915-023-01647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol Adv Ophthalmol. 2004;108(1):17–40. 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 31.Munteanu T, Noronha KJ, Leung AC, Pan S, Lucas JA, Schmidt TM. Light-dependent pathways for dopaminergic amacrine cell development and function. eLife. 2018;7:e39866. 10.7554/eLife.39866. [DOI] [PMC free article] [PubMed]
- 32.Wang L, Mao X. Role of retinal amyloid-β in neurodegenerative diseases: overlapping mechanisms and emerging clinical applications. Int J Mol Sci. 2021;22(5):2360. 10.3390/ijms22052360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiasseu M, Alarcon-Martinez L, Belforte N, et al. Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer’s disease. Mol Neurodegener. 2017;12(1):58. 10.1186/s13024-017-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popova E. Role of Dopamine in Retinal Function. In: Kolb H, Fernandez E, Jones B, Nelson R, eds. Webvision: the organization of the retina and visual system. Salt Lake City (UT): University of Utah Health Sciences Center. 2020.
- 35.Zhang NK, Zhang SK, Zhang LI, Tao HW, Zhang GW. Sensory processing deficits and related cortical pathological changes in Alzheimer’s disease. Front Aging Neurosci. 2023;15:1213379. 10.3389/fnagi.2023.1213379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kung HC, Lin KJ, Kung CT, Lin TK. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson’s disease. Biomedicines. 2021;9(8):918. 10.3390/biomedicines9080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrich MT, Oertel WH, Surmeier DJ, Geibl FF. Mitochondrial dysfunction in Parkinson’s disease - a key disease hallmark with therapeutic potential. Mol Neurodegener. 2023;18(1):83. 10.1186/s13024-023-00676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Çınar E, Tel BC, Şahin G. Neuroinflammation in Parkinson’s disease and its treatment opportunities. Balk Med J. 2022;39(5):318–33. 10.4274/balkanmedj.galenos.2022.2022-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merten N, Schultz AA, Walsh MC, et al. Psychological distress and well-being among sensory impaired individuals during COVID-19 lockdown measures. Ann Epidemiol. 2023;79:19–23. 10.1016/j.annepidem.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boussaty EC, Friedman RA, Million Veteran Program, Clifford RE. Hearing loss and tinnitus: association studies for complex-hearing disorders in mouse and man. Hum Genet. 2022;141(3–4):981–990. 10.1007/s00439-021-02317-9. [DOI] [PMC free article] [PubMed]
- 41.Tao Y, Zhao R, Yang B, Han J, Li Y. Dissecting the shared genetic landscape of anxiety, depression, and schizophrenia. J Transl Med. 2024;22(1):373. 10.1186/s12967-024-05153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javanmehr N, Saleki K, Alijanizadeh P, Rezaei N. Microglia dynamics in aging-related neurobehavioral and neuroinflammatory diseases. J Neuroinflammation. 2022;19(1):273. 10.1186/s12974-022-02637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seicol BJ, Lin S, Xie R. Age-related hearing loss is accompanied by chronic inflammation in the cochlea and the cochlear nucleus. Front Aging Neurosci. 2022;14:846804. 10.3389/fnagi.2022.846804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seicol BJ, Guo Z, Garrity K, Xie R. Potential uses of auditory nerve stimulation to modulate immune responses in the inner ear and auditory brainstem. Front Integr Neurosci. 2023;17:1294525. 10.3389/fnint.2023.1294525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallée A. Neuroinflammation in Schizophrenia: the key role of the WNT/β-Catenin pathway. Int J Mol Sci. 2022;23(5):2810. 10.3390/ijms23052810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu X, Jiang K, Chen R, et al. Identification of common stria vascularis cellular alteration in sensorineural hearing loss based on ScRNA-seq. BMC Genomics. 2024;25(1):213. 10.1186/s12864-024-10122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Zong S, Du P, et al. Role of the stria vascularis in the pathogenesis of sensorineural hearing loss: a narrative review. Front Neurosci. 2021;15:774585. 10.3389/fnins.2021.774585. Published 2021 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thulasiram MR, Ogier JM, Dabdoub A. Hearing function, degeneration, and disease: spotlight on the stria vascularis. Front Cell Dev Biol. 2022;10:841708. 10.3389/fcell.2022.841708. Published 2022 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. 2022;23(21):12906. 10.3390/ijms232112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14(1):30–8. 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CH, Kuo LT, Hung MJ, Cherng WJ. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J. 2002;144(2):275–81. 10.1067/mhj.2002.123843. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz RS, Burke A, Farb A, et al. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol. 2009;54(23):2167–73. 10.1016/j.jacc.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 53.Oron Y, Elgart K, Marom T, Roth Y. Cardiovascular risk factors as causes for hearing impairment. Audiol Neurootol. 2014;19(4):256–60. 10.1159/000363215. [DOI] [PubMed] [Google Scholar]
- 54.Keller J, Gomez R, Williams G, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22(4):527–36. 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knezevic E, Nenic K, Milanovic V, Knezevic NN. The role of cortisol in chronic stress, neurodegenerative diseases, and psychological disorders. Cells. 2023;12(23):2726. 10.3390/cells12232726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gianotti L, Belcastro S, D’Agnano S, Tassone F. The stress axis in obesity and diabetes mellitus: an update. Endocrines. 2021;2(3):334–47. 10.3390/endocrines2030031. [Google Scholar]
- 57.Lyra E Silva N de M, Lam MP, Soares CN, Munoz DP, Milev R, De Felice FG. Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front Psychiatry. 2019;10:57. 10.3389/fpsyt.2019.00057. [DOI] [PMC free article] [PubMed]
- 58.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 59.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willemen HLDM, Santos Ribeiro PS, Broeks M, et al. Inflammation-induced mitochondrial and metabolic disturbances in sensory neurons control the switch from acute to chronic pain. Cell Rep Med. 2023;4(11):101265. 10.1016/j.xcrm.2023.101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martins B, Pires M, Ambrósio AF, Girão H, Fernandes R. Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction. J Biomed Sci. 2024;31(1):48. 10.1186/s12929-024-01036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Negro I, Pauletto G, Verriello L, et al. Uncovering the genetics and physiology behind optic neuritis. Genes. 2023;14(12):2192. 10.3390/genes14122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ojaghi H, Poorsheykhian S, Najafi A, Iranpour S. The role of blood related inflammatory factors on age-related macular degeneration (AMD). Immun Ageing A. 2024;21(1):35. 10.1186/s12979-024-00440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YC, Xu K. Macrophage-related immune responses in inner ear: a potential therapeutic target for sensorineural hearing loss. Front Neurosci. 2023;17:1339134. 10.3389/fnins.2023.1339134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X, Zhang Q, Zheng R. The interplay between oxidative stress and autophagy in chronic obstructive pulmonary disease. Front Physiol. 2022;13:1004275. 10.3389/fphys.2022.1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raby KL, Michaeloudes C, Tonkin J, Chung KF, Bhavsar PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol. 2023;14:1201658. 10.3389/fimmu.2023.1201658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao P, Sun T, Lyu C, Liang K, Du Y. Cell mediated ECM-degradation as an emerging tool for anti-fibrotic strategy. Cell Regen Lond Engl. 2023;12(1):29. 10.1186/s13619-023-00172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Dev Camb Engl. 2006;133(15):2995–3004. 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed M, Wong EYM, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012;22(2):377–90. 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phelan PJ, Rheault MN. Hearing loss and renal syndromes. Pediatr Nephrol Berl Ger. 2018;33(10):1671–83. 10.1007/s00467-017-3835-9. [DOI] [PubMed] [Google Scholar]
- 71.Kleppel MM, Santi PA, Cameron JD, Wieslander J, Michael AF. Human tissue distribution of novel basement membrane collagen. Am J Pathol. 1989;134(4):813–25. [PMC free article] [PubMed] [Google Scholar]
- 72.Greenberg D, Rosenblum ND, Tonelli M. The multifaceted links between hearing loss and chronic kidney disease. Nat Rev Nephrol. 2024;20(5):295–312. 10.1038/s41581-024-00808-2. [DOI] [PubMed] [Google Scholar]
- 73.Tanriover C, Copur S, Mutlu A, et al. Early aging and premature vascular aging in chronic kidney disease. Clin Kidney J. 2023;16(11):1751–65. 10.1093/ckj/sfad076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frank CR, Xiang X, Stagg BC, Ehrlich JR. Longitudinal associations of self-reported vision impairment with symptoms of anxiety and depression among older adults in the United States. JAMA Ophthalmol. 2019;137(7):793–800. 10.1001/jamaophthalmol.2019.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang AR, Deal JA, Rebok GW, Pinto JM, Waite L, Lin FR. Hearing impairment and loneliness in older adults in the United States. J Appl Gerontol Off J South Gerontol Soc. 2021;40(10):1366–71. 10.1177/0733464820944082. [DOI] [PubMed] [Google Scholar]
- 76.Li CM, Zhang X, Hoffman HJ, Cotch MF, Themann CL, Wilson MR. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol-- Head Neck Surg. 2014;140(4):293–302. 10.1001/jamaoto.2014.42. [DOI] [PMC free article] [PubMed]
- 77.Chen DS, Betz J, Yaffe K, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(5):654–61. 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendrinos E, Machinis TG, Pournaras CJ. Ocular ischemic syndrome. Surv Ophthalmol. 2010;55(1):2–34. 10.1016/j.survophthal.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 79.Ivanova E, Alam NM, Prusky GT, Sagdullaev BT. Blood-retina barrier failure and vision loss in neuron-specific degeneration. JCI Insight. 2019;5(8):e126747, 126747. 10.1172/jci.insight.126747. [DOI] [PMC free article] [PubMed]
- 80.Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, Dubno JR. White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol JARO. 2013;14(3):425–33. 10.1007/s10162-013-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belal A. Pathology of vascular sensorineural hearing impairment. Laryngoscope. 1980;90(11 Pt 1):1831–9. 10.1288/00005537-198011000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Watson N, Ding B, Zhu X, Frisina RD. Chronic inflammation - inflammaging - in the ageing cochlea: a novel target for future presbycusis therapy. Ageing Res Rev. 2017;40:142–8. 10.1016/j.arr.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Fu X, Li Y, et al. Macrophage-mediated immune response aggravates hearing disfunction caused by the disorder of mitochondrial dynamics in cochlear hair cells. Hum Mol Genet. 2023;32(7):1137–51. 10.1093/hmg/ddac270. [DOI] [PubMed] [Google Scholar]
- 84.Ozawa Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020;37:101779. 10.1016/j.redox.2020.101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5(6):251–65. 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shadrina MI, Slominsky PA, Limborska SA. Molecular mechanisms of pathogenesis of Parkinson’s disease. Int Rev Cell Mol Biol. 2010;281:229–66. 10.1016/S1937-6448(10)81006-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Part 1 includes introduction to The China Health and Retirement Longitudinal Study (CHARLS) and basic characters evaluation methods. Part 2 includes MR methods. Table. S1 Data sources for exposures and outcomes. Part 3 includes 22 tables, and 56 figures related to the results of cohort studies and Mendelian randomization studies. Table. S2-S4 Number and proportion of individuals at five different levels of auditory acuity/ distance visual acuity/ near visual acuity in 2011 and 2013. Table. S5-S7 Estimated burden of chronic diseases and multimorbidity attributable to HI/VI/DSI and HI/VI/DSI transition patterns. Table.S8-S10 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort using multiple imputation approach. Table.S11-S13 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk in the CHARLS cohort using the propensity scores of overlap weighting. Table.S14-S16 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk after excluding those with outcomes occurring within the first year in the CHARLS cohort. Table.S17-S19 Sensitivity Analysis: Association of HI/VI/DSI in 2013 and HI/VI/DSI transition patterns from 2011 to 2013 with 10 chronic diseases and multimorbidity new-onset risk after adjusted for the use of hearing/visual aids. Table.S20 Genetic variants that were used as instruments for VI. Table.S21 MR analysis with random-effects IVW, MR-Egger, weighted median for association between VI/HI and 17 outcomes, MR-Egger intercept/MRPRESSO test for horizontal pleiotropy, and heterogeneity tests with Cochran’s Q statistic. Table.S22 Genetic variants that were used as instruments for HI. Fig. S1 Distribution of Hearing and Vision Levels Reported in 2011 and 2013 (A), Distribution of Hearing Levels B) Population Distribution of HI Dynamic Change Patterns C) Distribution of Distance Vision Levels D) Distribution of Near Vision Levels E) Population Distribution of VI Dynamic Change Patterns. Fig. S2 Kaplan-Meier plots of the cumulative incidence to new-onset chronic diseases and multimorbidity in sub-cohorts, stratified by whether HI/VI/DSI in 2013. Fig. S3 PAF%(95%CI) for chronic diseases and multimorbidity due to HI/VI/DSI & HI/VI/DSI transition patterns. Fig.S4-S7 Sensitivity analysis heatmap: impact of data imputation, PS matching, first-year event exclusion, and sensory aid adjustment. Fig. S8 Forest plots of the risk effects (OR, 99.896%CI) of genetically predicted VI/HI on chronic diseases. Fig.S9-S56 Scatter plot for the causal association between HI/VI and 24 disease status and leave-one-out tests.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.