Abstract
Background
Non-communicable chronic disease is a major contributor to morbidity and mortality with potentially modifiable lifestyle factors. In women, the menopausal transition modifies women’s risk of chronic disease, and pregnancy-related complications have been highlighted as female-specific risk factors. Later reproductive years, before onset of menopause, may represent a window of opportunity for promotion of lifestyle modifications. The aim of this scoping review is to investigate which interventions promoting lifestyle modifications in women of later reproductive years may influence cardiometabolic and bone disease.
Methods
A search of three electronic databases (PubMed, Embase, CINAHL) in the English language was performed in January 2024. Eligible studies included women aged 40–55 participating in interventions focusing on lifestyle modification. Studies reporting outcomes related to cardiometabolic disease, bone disease or body composition were eligible for inclusion.
Results
Improvements in body composition occurred following interventions focusing on aerobic physical activity. Interventions focusing on health promotion and education, incorporating both dietary and physical activity modifications, prevented weight gain and improved cardiometabolic outcomes. Interventions incorporating elements of behavioural theories enhanced patient-motivated lifestyle modifications, with effects on body composition and cardiometabolic outcomes.
Conclusions
Lifestyle modifications in later reproductive years have the potential to influence cardiometabolic and bone disease. Our findings reinforce the benefits of regular aerobic physical activity, as well as health education, for improving body composition and lipid profile. This information could contribute to the development of clinical guidelines for the prevention of chronic disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-025-00908-1.
Keywords: Cardiometabolic disease, Bone disease, Women, Later reproductive years, Lifestyle modifications
Background
The global burden of non-communicable, chronic disease (NCDs) is steadily increasing, contributing to 41 million deaths each year, and up to 74% of all global deaths [1]. Cardiovascular disease in particular, accounts for the largest proportion of deaths attributable to NCDs, up to 17.9 million people annually [1]. Additionally, musculoskeletal health, including osteoporosis and sarcopenia, is now recognized as a leading contributor to disability worldwide, across multiple age brackets [2], and may also be associated with development of chronic disease [3]. NCDs tend to be of chronic duration and slow progress, sharing common underlying modifiable risk factors including unhealthy diet, low levels of physical activity, and tobacco use [1], which may be the target of lifestyle modifications [4]. Furthermore, metabolic risk factors may contribute to changes in metabolic status, including hypertension, overweight or obesity, hyperglycaemia or hyperlipidaemia which increase the risk of development of NCDs [1].
While the incidence of cardiometabolic disorders continues to rise among both men and women, likely attributable to obesity-related metabolic risk factors, female-specific risk factors may contribute to the rising incidence of cardiometabolic disorders among younger women [5]. Notable differences include pregnancy-related complications such as hypertensive disorders in pregnancy and gestational diabetes [6, 7]. Women who develop pre-eclampsia in pregnancy have up to a 2–5 fold increased risk of developing cardiovascular disease [6], while women who develop gestational diabetes have an 8–10 fold increased risk of developing type 2 diabetes mellitus [8], and a two-fold increased risk of cardiovascular events [9]. Furthermore, hormonal influences are key in the progression of cardiometabolic disease, where on average women are affected by cardiometabolic disease approximately 7–10 years later than men, largely due to the protective effects of oestrogen during the reproductive years [6]. Menopause, a state characterised by oestrogen deficiency, is known to change the cardiometabolic risk profile for women, namely through alterations in lipid profile and increases in body mass [10]. Additionally, physical inactivity and lower hormone secretion in middle-aged women can also lead to decreased lean mass, muscle strength, and bone mass [11].
While female-specific risk factors for NCDs are acknowledged, there remains a paucity of data as to how best to implement risk reduction strategies. Despite increasing evidence, pregnancy-related complications are often not adequately incorporated into risk factor screening for NCDs, and timing for optimal intervention remains unclear [7]. The later reproductive years represents the stage in a woman’s life where child-bearing is complete, however the menopausal transition has not yet been reached [12], typically around the ages of 40–55. In this stage, women may begin to experience menstrual irregularities, but the onset of menopause has not yet occurred [12]. Therefore, we propose that the later reproductive years may represent a unique timepoint in a woman’s life to focus on lifestyle modifications before the onset of menopause.
The aim of the current scoping review is to identify and summarise interventions relating to lifestyle modifications which have been trialled in women of later reproductive years, that may have the potential to influence cardiometabolic and bone disease.
Methods
Study design
A scoping review was performed to identify types of lifestyle modification interventions that have been trialled, which may have potential to improve overall cardiometabolic and bone health among women in the later reproductive years. This scoping review was conducted based on the methodological frameworks of Arksey and O’Malley [13], and the Joanna Briggs Institute [14], while following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [15]. A scoping review protocol was developed internally but not registered publicly.
Eligibility criteria
Eligibility was determined using PICO framework (Table 1).
Table 1.
PICO framework
| Population | Women aged 40–55 who had not reached menopause |
|---|---|
| Intervention | Interventions focusing on lifestyle modification, e.g. physical activity, diet, supplements or behaviour change techniques |
| Comparator | No comparator |
| Outcome | Body composition, metabolic parameters (blood pressure, blood lipid profile, glucose and insulin homeostasis), inflammatory markers, bone and muscle health, or bone mineral density |
Search strategy
The search strategy was developed by the research team, in collaboration with the medical librarian at the University College Dublin. The search consisted of a combination of Medical Subject Headings (MeSH) and focused keywords. The search was performed in January 2024 across three databases (PubMed, EMBASE and CINAHL). The full PubMed search strategy can be found in supplementary Table 1 (Additional File 1), and was similarly applied to the other electronic databases. Searches were limited to human studies, the English language, and randomized controlled trials (RCTs) or quasi-experimental clinical trials, as these represent the highest quality forms of evidence for interventions. There was no time limitation placed.
Study selection & data management
All records were exported into an EndNote™ (version 20, Clavariate, Philadelphia, USA) library, then uploaded to Covidence software (Veritas Health Innovation, Melbourne, Australia) and duplicates removed. Articles were screened by title and abstract, followed by full texts by two independent researchers (KD and GD), according to pre-determined eligibility criteria, with input from a third researcher (FMcA) to reach agreement in the event of a discrepancy. Manual searching of reference lists was also performed.
Data extraction and synthesis
Once eligible articles were finalized by the research team, relevant data was systematically extracted by the primary researcher into a standardized data extraction form in Microsoft Excel. The following variables were extracted: year, country, study design, subject characteristics and sample size, attrition, intervention description and duration, follow-up duration (if any), and primary findings. Descriptive statistics were generated using Microsoft Excel, and studies were classified according to primary outcome and intervention focus. Findings are described using a narrative synthesis approach.
Results
Selection of articles
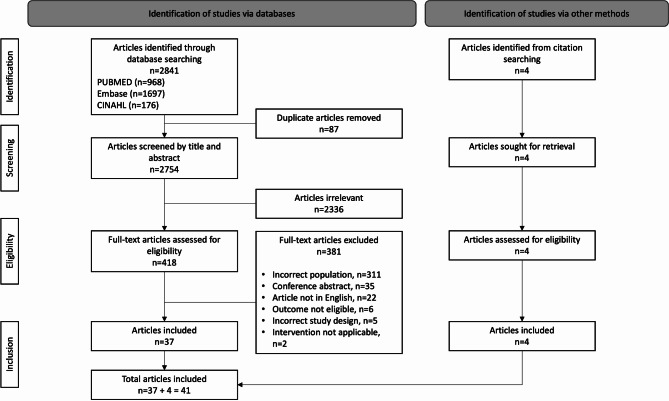
The search strategy identified 2841 citations across three databases (Fig. 1). After removal of duplicates and title abstract screening, 418 full-text articles were retrieved and reviewed. Of these citations, 37 were eligible for inclusion, with 4 further articles identified from hand-searching reference lists, ultimately yielding 41 publications for inclusion. A detailed summary of all interventions is presented in supplementary Table 2 (Additional File 2).
Fig. 1.
PRISMA-ScR flow chart. PRISMA-ScR flow diagram of study selection
Study characteristics
Table 2 details the study characteristics of the 41 studies, representing results from 25 RCTs (61.0%) and 16 quasi-experimental clinical studies (39.0%). There were 33 primary interventions, 7 articles detailing follow-up studies of primary interventions, and one secondary analysis of a primary intervention. The majority of studies (n = 15, 36.6%) originated from Asia, followed by North America (n = 11, 26.8%), Africa & The Middle East (n = 7, 17.1%), Australia (n = 3, 7.3%), Europe (n = 3, 7.3%), and Latin & South America (n = 2, 4.9%).
The majority of studies focused on healthy participants (n = 19, 46.3%), or participants with overweight/obesity and no medical co-morbidities (n = 13, 31.7%). A small number of studies (n = 4, 9.8%) examined women with a previous diagnosis of a metabolic co-morbidity, such as type 2 diabetes mellitus or dyslipidaemia, and 5 studies (12.2%) only specified the women were experiencing perimenopausal symptoms.
The interventions described were of variable duration and follow-up; 36.6% of interventions (n = 15) lasted for 8 weeks or less, 29.23% (n = 12) of interventions lasted for 9–12 weeks, 26.8% (n = 11) of interventions lasted for 13–26 weeks, with only 3 studies (7.3%) lasting 27–52 weeks in duration. The majority of studies described the intervention only (n = 28, 68.3%). One primary intervention included a follow-up period of 6 months [16]. Another primary intervention included a 12 month maintenance period, and was discussed in 3 studies [17–19]. The “40-something RCT” included follow-up to 24 months, and was reported across 3 studies [20–22]. The “Women’s Healthy Lifestyle Project” followed up participants to 54 months, and results were presented across 4 publications [23–26] (See Table 2).
Table 2.
Study characteristics
| Characteristics | Number of studies (%) | |
|---|---|---|
| Study characteristics | Primary intervention | 33 (80.5%) |
| Follow-up of primary intervention | 7 (17.1%) | |
| Secondary analysis | 1 (2.4%) | |
| Randomized controlled trial | 25 (61.0%) | |
| Quasi-experimental clinical trial | 16 (39.0%) | |
| Study participants | Healthy | 19 (46.3%) |
| People with overweight or obesity | 13 (31.7%) | |
| Peri-menopausal only | 5 (12.2%) | |
| Metabolic co-morbidity | 4 (9.8%) | |
| Sample size | ≤ 10 | - |
| 11–50 | 12 (29.3%) | |
| 51–99 | 17 (41.5%) | |
| ≥ 100 | 12 (29.23%) | |
| Duration of intervention (weeks) | ≤ 8 weeks | 15 (36.6%) |
| 9–12 weeks | 12 (29.3%) | |
| 13–26 weeks | 11 (26.8%) | |
| 27–52 weeks | 3 (7.3%) | |
Interventions with effects on body composition
Of 24 interventions with effects on body composition, 41.7% (n = 10/24) focused on physical activity, 16.7% (n = 4/24) focused on use of supplements, 2 primary interventions focused on behaviour change techniques with follow-up of participants in a further 4 studies, and 2 primary interventions focused on health promotion and education with follow-up of participants in a further 2 studies. Sample sizes ranged from 16 to 300, and duration of intervention ranged from 8 weeks to 54 months (Table 3).
Table 3.
Summary of interventions with effects on body composition
| Author | Primary focus of intervention | Key findings |
|---|---|---|
| Aneis et al. [28]b | Physical activity | Aerobic +/- strength training for 12 weeks reduced BMI and waist circumference |
| Chung et al. [29]b | Physical activity | Multiple short exercise sessions reduced waist circumference |
| Cussler et al. [17]d | Behaviour change techniques and follow-up | Following the “Healthy Weight for Life” program, there was no significant difference in weight re-gain or energy expenditure in women who utilized the internet for ongoing support |
| El Shebini et al. [86]c | Supplements | Turmeric and ginger supplementation along with a hypocaloric diet for 8 weeks improved body composition |
| El Shebini et al. [87]d | Supplements | Foeniculum vulgare (fennel powder) and Salvia hispanica (chia seeds) supplementation along with a hypocaloric diet for 8 weeks improved body composition |
| Hao et al. [27]c | Health promotion & education | Routine health education centred on DASH dietary principles and increasing physical activity reduced body mass |
| Hollis et al. [20]b | Behaviour change techniques and follow-up | The effect of “The 40-something RCT” was maintained up to 24 months following the intervention. Encouraging women to take at least 10,000 steps and consume 5 servings of vegetables per day accounted for the weight loss observed |
| Karatrantou et al. [30]c | Physical activity | Performance of low-impact aerobic dance and calisthenics for 3 months decreased body fat, improved body composition, and increased indices of overall fitness |
| Krishnan et al. [31]b | Physical activity | Aerobic exercise performed 3 times per week and resistance exercise performed 3 times per week for 6 months induced body weight and fat loss |
| Moaty et al. [88]c | Supplements | Turmeric and ginger supplementation along with a hypocaloric diet for 8 weeks decreased anthropometric measurements |
| Moslehi et al. [38]c | Supplements | 250 mg magnesium oxide taken daily for 8 weeks reduced body fat percentage and fat mass |
| Oh et al. [32]a | Physical activity | Aerobic exercise of vigorous or moderate intensity for 8 weeks decreased weight and body fat |
| Park et al. [35]b | Physical activity | Aerobic exercise only decreased subcutaneous and visceral fat, whereas aerobic +/- resistance training for 24 weeks improved blood lipid profile |
| Pilch et al. [33]a | Physical activity | Aerobic exercise alternating high-intensity and low-intensity movements for 12 weeks improved body composition |
| Ribeiro et al. [34]d | Physical activity | Aerobic training for 12 weeks reduced weight and waist circumference, whereas a pedometer-based intervention had greatest impact on increasing overall step count |
| Simkin-Silverman [24]d | Health promotion & education | “Women’s Healthy Lifestyle Project”, an intervention emphasising lifestyle modifications to lower cholesterol and increase physical activity, demonstrated significant improvements in lipid profile and modest weight loss after a 20 week intervention |
| Simkin-Silverman [26]d | Health promotion & education and follow-up | 18 months after the “Women’s Healthy Lifestyle Project”, those who participated in the intervention demonstrated greater levels of physical activity, lower caloric intake, and lower BMI |
| Simkin-Silverman [25]d | Health promotion & education and follow-up | 54 months after the “Women’s Healthy Lifestyle Project”, women in the intervention group had maintained weight loss and remained more physically active |
| Teixeira et al. [18]d | Behaviour change techniques | “Healthy Weight for Life” program, based off cognitive and behavioural strategies to motivate progressive changes in lifestyle, over 16 weeks resulted in significant improvements in body weight and body composition |
| Teixeira et al. [19]d | Behaviour change techniques and follow-up | Following the “Healthy Weight for Life” program, 1 year of ongoing support did not influence change in weight, compared with no additional contact from the study group |
| Tsai et al. [36]c | Physical activity | Muscle/tendon change classic (MTCC) qigong performed daily for 8 weeks reduced waist-to-hip ratio and BMI |
| Williams et al. [79]b | Behaviour change techniques | “The 40-something RCT” utilized a lifestyle motivation centred upon motivational interviewing to promote behaviour change in relation to lifestyle changes to improve weight control over 3 months. Women in the motivational interviewing group lost more weight during the intervention period |
| Williams et al. [21]b | Behaviour change techniques and follow-up | Women in the motivational interviewing group in “The 40-something RCT” lost significantly more weight during the intervention period, regained some of the weight by 18 months, but maintained a stable weight up to 24 months post-intervention |
| Zhang et al. [42]d | Physical activity | Aerobic exercise performed as “walking with strides” improved blood lipid profile |
Note: Behaviour change techniques included social, cognitive or behavioural theory components as part of the lifestyle modification intervention. Health education and promotion combined multiple lifestyle elements, such as dietary modifications and physical activity, into a composite lifestyle intervention. a Sample size ≤ 20, b Sample size 21–50, c Sample size 51–99, d Sample size ≥ 100. Abbreviations– BMI: body mass index; RCT: randomized controlled trial; MTCC: Muscle/tendon change classic
The “Women’s Healthy Lifestyle Project” successfully prevented weight gain and reduced waist circumference over a period of 5 years [23–26], during the menopausal transition, when women typically experience increased body mass. The “40-something RCT” also prevented weight gain over 24 months of the menopausal transition by employing motivational interviewing techniques with a focus on obesity prevention [20–22]. The “Healthy Weight for Life” intervention made use of cognitive and behavioural strategies to improve adherence with the lifestyle modifications, and also demonstrated significant reductions in weight, body mass index (BMI), fat mass and waist circumference [18, 19]. Hao et al. demonstrated that an intervention combining changes in diet and physical activity reduced body mass [27].
As well, increased physical activity was noted to improve body composition and promote weight loss. Any type of aerobic and/or strength training induced significant improvements in body composition [28–34]. Most studies reported a global improvement following intervention, with no significant differences between aerobic training alone versus aerobic training combined with strength and/or resistance training [28, 29, 31, 32]. Interestingly, multiple short sessions of exercise were more effective in reducing waist circumference when compared with a single prolonged session of exercise [29], and combining aerobic training with resistance training was more effective in reducing subcutaneous and visceral adipose tissue when compared to aerobic training alone [35]. Practicing MTCC qigong daily over the course of 8 weeks significantly reduced waist-to-hip ratio and BMI [36].
The only supplement noted to improve body composition was magnesium [37, 38]. A dose of 250 mg daily for 8 weeks resulted in lower body fat percentage and fat mass [37], along with increased lean mass [38] when compared to baseline values.
Interventions with effects on cardiometabolic outcomes
Of 22 interventions with effects on cardiometabolic outcomes, 54.5% (n = 12/22) focused on physical activity, 22.7% (n = 5/22) focused on use of supplements, while 3 primary interventions focused on health promotion and education with follow-up of participants in a further 2 studies. Sample sizes ranged from 20 to 489, with duration of intervention ranging from 8 weeks to 54 months (Table 4).
Table 4.
Summary of interventions with effects on cardiometabolic outcomes
| Author & reference | Primary focus of intervention | Key findings |
|---|---|---|
| Aneis et al. [28]b | Physical activity | Aerobic +/- strength training for 12 weeks improved blood lipid profile and insulin sensitivity |
| Beak et al. [89]a | Physical activity | Combined aerobic and resistance training for 8 weeks improved inflammation markers |
| Chung et al. [29]b | Physical activity | Single prolonged exercise session improved blood lipid profile, whereas multiple short exercise sessions reduced blood glucose |
| Costa et al. [39]b | Physical activity | Water-based aerobic training for 12 weeks improved blood lipid profile |
| El Shebini et al. [86]c | Supplements | Turmeric and ginger supplementation along with a hypocaloric diet for 8 weeks improved fasting blood glucose and serum lipid profile |
| El Shebini et al. [87]d | Supplements | Foeniculum vulgare (fennel powder) and Salvia hispanica (chia seeds) supplementation along with a hypocaloric diet for 8 weeks improved blood pressure. Fennel powder improved fasting glucose and lipid profile more than chia seeds |
| Farahati et al. [40]b | Physical activity | High-intensity interval training and moderate-intensity continuous training for 12 weeks both improved markers of vascular function and blood lipid profile |
| Hao et al. [27]c | Health promotion & education | Routine health education centred on DASH dietary principles and increasing physical activity improved blood lipid profile |
| Hassan et al. [16]c | Health promotion & education | “Women Wellness Workshop” in the primary care setting significantly reduced blood pressure and increased daily energy expenditure |
| Kim et al. [41]b | Physical activity | Dynamic exercise (combining low-impact aerobics and weight-bearing exercise) for 8 weeks reduced total cholesterol levels |
| Krishnan et al. [31]b | Physical activity | Aerobic exercise performed 3 times per week and resistance exercise performed 3 times per week for 6 months improved insulin sensitivity |
| Kuller et al. [23]d | Health promotion & education and follow-up | 54 months after the “Women’s Healthy Lifestyle Project”, there was a significantly smaller increase in LDL cholesterol |
| Moaty et al. [90]c | Supplements | Hyphaenae thebaica (doum flour) supplement in combination with a hypocaloric diet for 8 weeks decreased lipid accumulation products |
| Moaty et al. [88]c | Supplements | Turmeric and ginger supplementation along with a hypocaloric diet for 8 weeks improved lipid profile and insulin sensitivity |
| Obaya et al. [43]c | Physical activity | Aerobic exercise with slow deep breathing and mindfulness techniques for 6 weeks reduced cortisol levels and improved fasting glucose values in type 2 diabetic women |
| Oh et al. [32]a | Physical activity | Aerobic exercise of vigorous or moderate intensity for 8 weeks improved blood lipid profile |
| Park et al. [35]b | Physical activity | Aerobic exercise only decreased subcutaneous and visceral fat, whereas aerobic +/- resistance training for 24 weeks improved blood lipid profile |
| Pilch et al. [33]a | Physical activity | Aerobic exercise alternating high-intensity and low-intensity movements for 12 weeks improved blood lipid profile |
| Simkin-Silverman [24]d | Health promotion & education | “Women’s Healthy Lifestyle Project”, an intervention emphasising lifestyle modifications to lower cholesterol and increase physical activity, demonstrated significant improvements in lipid profile and modest weight loss after a 20 week intervention |
| Simkin-Silverman [26]d | Health promotion & education and follow-up | 18 months after the “Women’s Healthy Lifestyle Project”, those who participated in the intervention demonstrated lower LDL cholesterol levels |
| Washburn et al. [44]b | Supplements | Soya protein containing phytoestrogen consumed over 6 weeks improved lipid profile and diastolic blood pressure |
| Zhang et al. [42]d | Physical activity | Aerobic exercise performed as “walking with strides” reduced weight, BMI, and waist circumference |
Note: Behaviour change techniques included social, cognitive or behavioural theory components as part of the lifestyle modification intervention. Health education and promotion combined multiple lifestyle elements, such as dietary modifications and physical activity, into a composite lifestyle intervention. a Sample size ≤ 20, b Sample size 21–50, c Sample size 51–99, d Sample size ≥ 100. Abbreviations–RCT: randomized controlled trial; LDL– low density lipoprotein
Similar to body composition, any form of aerobic exercise in previously sedentary women demonstrated significant improvements in lipid profile [29, 32, 33, 35, 39–42], regardless of intensity [32] or form of aerobic exercise [39, 41, 42]. A single prolonged exercise session was more effective in improving lipid profile, compared with multiple short sessions of exercise [29]. Water aerobics [39] and “walking with strides” [42] also improved serum lipid profile.
Insulin sensitivity and markers of glucose homeostasis were improved following multiple primary interventions [28, 31, 43]. Aerobic exercise in combination with resistance training significantly improved insulin sensitivity [28, 31], while multiple short sessions of aerobic exercise lowered serum glucose levels in healthy, sedentary, pre-menopausal women [29]. Combining aerobic exercise with slow, deep breathing and mindfulness techniques improved circulating cortisol levels and fasting glucose values in pre-menopausal women with type 2 diabetes mellitus [43].
The “Women’s Healthy Lifestyle Project” was able to mitigate the rise in low density lipoprotein (LDL) cholesterol which is known to occur during the menopausal transition. Women in the intervention group had significantly smaller increases in LDL cholesterol levels throughout the intervention and maintenance period [23–26]. Additionally, the intervention by Hao et al., which combined education regarding diet using the DASH principles and the benefits of exercise, also demonstrated significant improvements in lipid profile, and successful reduction of blood pressure [27].
Soya supplements containing phytoestrogens demonstrated a reduction in total cholesterol and LDL cholesterol, in addition to a reduction in diastolic blood pressure, in the absence of any changes in weight [44].
Interventions with effects on bone health
Of 5 interventions with effects on bone health, 1 intervention focused on physical activity, 3 interventions focused on use of supplements, and 1 was a secondary analysis of an intervention focused on health promotion and education. Sample sizes ranged from 58 to 535, with interventions lasting 8 weeks in duration (Table 5).
Table 5.
Summary of interventions with effects on bone health outcomes
| Author & Reference | Primary Focus of Intervention | Key Findings |
|---|---|---|
| Kheyruri et al. [45]c | Supplements | Co-supplementation of 50,000 units of vitamin D weekly and 250 mg magnesium oxide daily for 8 weeks improved muscle function and handgrip strength, but had no effect on knee extension strength or body composition |
| Moaty et al. [90]c | Supplements | Hyphaenae thebaica (doum flour) supplement in combination with a hypocaloric diet for 8 weeks decreased bone-specific alkaline phosphatase |
| Moslehi et al. [38]c | Supplements | 250 mg magnesium oxide taken daily for 8 weeks increased lean mass and decreased fat mass, without alterations in muscle strength or functional mobility |
| Salamone et al. [46]d | Health promotion & education | Women who lost weight as part of the “Women’s Healthy Lifestyle Project” demonstrated 3 times greater rate of bone mineral density loss |
| Tsai et al. [36]c | Physical activity | Muscle/tendon change classic (MTCC) qigong performed daily for 8 weeks improved muscle endurance |
Note: Behaviour change techniques included social, cognitive or behavioural theory components as part of the lifestyle modification intervention. Health education and promotion combined multiple lifestyle elements, such as dietary modifications and physical activity, into a composite lifestyle intervention. a Sample size ≤ 20, b Sample size 21–50, c Sample size 51–99, d Sample size ≥ 100. Abbreviations– MTCC: Muscle/tendon change classic
The practice of traditional MTCC qigong was found to increase muscle endurance [36]. The co-supplementation of vitamin D and magnesium was associated with increased handgrip strength and improved muscle function [45], whereas magnesium supplementation alone was associated with increased lean mass without alterations in muscle strength or functional mobility [38]. Lastly, weight loss during the “Women’s Healthy Lifestyle Program” was associated with a 3-times higher rate of loss of bone mineral density [46].
Interventions with equivocal outcomes
A number of interventions demonstrated uncertain significance, where 1 primary intervention focused on physical activity, and 4 interventions trialled use of supplements (Table 6).
Table 6.
Summary of interventions with equivocal outcomes
| Author & Reference | Primary Focus of Intervention | Key Findings |
|---|---|---|
| Abiri et al. [50]c | Supplements | 1000 units of oral vitamin D supplement daily for 12 weeks did not significantly change weight, BMI or functional muscle strength |
| Chinnappan et al. [47]c | Supplements | “NuFemme”, a supplement containing Labisia pumila and Eurycoma longifolia at a dose of 400mg L. pumila and 100mg E. longifolia for 24 weeks demonstrated a non-significant trend towards improving lipid profile, with no significant difference in markers of bone health |
| Jamshidi et al. [51]c | Supplements | Vitamin D supplementation of 50,000 units weekly, in combination with a synbiotic (combination of prebiotic and probiotic) for 8 weeks demonstrated a decreasing trend in body fat percentage, fat mass and visceral fat area |
| Luzzi et al. [48]b | Supplements | “Pycnogenol”, a supplement derived from Pinus pinaster, consumed at a dose of 100 mg daily for 8 weeks normalized borderline metabolic risk factors |
| Mortimer et al. [91]a | Physical activity | Blood pressure was reduced in all participants in response to laboratory monitoring for 5 days, regardless of performance of isometric handgrip training |
Note: Behaviour change techniques included social, cognitive or behavioural theory components as part of the lifestyle modification intervention. Health education and promotion combined multiple lifestyle elements, such as dietary modifications and physical activity, into a composite lifestyle intervention. a Sample size ≤ 20, b Sample size 21–50, c Sample size 51–99, d Sample size ≥ 100. Abbreviations– BMI: body mass index
Compounds which exerted hormone-like effects were explored as potential supplements for modifying menopausal symptoms and metabolic risk profile. An herbal supplement containing Labisia pumila and Eurycoma longifolia (“NuFemme”) was examined as an alternative to hormonal replacement therapy [47], and demonstrated a trend towards improved lipid profile, with no improvement in markers of bone health. In this study, 119 participants initiated the study, with an attrition rate of 44 participants, which may explain the equivocal results [47]. Additionally, the use of an extract derived from Pinus pinaster (“Pycnogenol”) normalized borderline risk factors for metabolic syndrome in perimenopausal women that were present prior to initiation of the supplement [48]. Due to the age of the participants, the small sample size, and the fact use of the supplement was completely voluntary, results were considered equivocal [49].
The effect of vitamin D alone was explored [50], but did not significantly alter body composition for functional muscle strength. Vitamin D was also explored with co-supplementation with a synbiotic (combination of prebiotic and probiotic to improve host gut microflora), resulting in a trend towards reduced body fat percentage and visceral fat area [51]. These interventions were of relatively short duration (8 weeks), which may limit the ability to observe significant results.
Discussion
Main findings
The purpose of this scoping review was to identify and examine the existing literature regarding lifestyle modifications trialled among women of later reproductive age which may have the potential to improve cardiometabolic and bone health. In this scoping review, 41 studies were eligible for inclusion. Improvements in body composition and anthropometry were most notable following interventions which focused on aerobic physical activity of any nature or intensity. Additionally, interventions which focused on health promotion and education incorporating both dietary and physical activity modifications appeared successful in preventing weight gain. Interventions which incorporate elements of behavioural, social and cognitive theories were also noted to enhance patient-motivated lifestyle modifications, with effects on body composition and cardiometabolic outcomes. Supplements were explored in relation to their effects on body composition, cardiometabolic and bone outcomes, however they did not appear to have consistent benefits on body composition or lipid profile, but may have some effects on bone health.
Mechanisms
The later reproductive years mark the beginning of the menopausal transition, which spans until the early post-menopausal period [52]. During this time, complex endocrinology potentially influences cardiometabolic and bone metabolism. Fluctuations in oestrogen, follicle stimulating hormone (FSH) and luteinizing hormone (LH) during this time period have been linked to negative effects on bone and cardiometabolic health [52].
Across the menopausal transition, it has been noted there is a worsening in lipid profile, namely through increases in total cholesterol and LDL cholesterol [53, 54]. Not only is weight gain particularly prevalent during the mid-life years, a change in body composition and fat distribution is noted, with a shift away from lean mass and greater central obesity noted following menopause [55]. Physical activity improves dyslipidaemia and markers of chronic inflammation across the menopausal transition, however the exact mechanisms underpinning these observations remains unknown [56]. Additionally, the definition of a ‘healthy diet’ is constantly evolving and changing based on emerging evidence. Alterations in dietary patterns are postulated to regulate pathways involved in glucolipid and energy balance, suppression of inflammation and alteration of the gut microbiome [57]. Lifestyle modifications which target metabolic changes that occur across the menopausal transition may have potential to modulate later-life cardiometabolic risk.
The three years surrounding the final menstrual period are characterised by a rapid phase of bone loss, with on average 2.5% decline in bone mineral density of the lumbar spine each year [58]. It is postulated that these changes are driven by alterations in oestradiol and FSH levels, altering the balance of bone turnover markers and changing the composition of the bone [59]. Other important factors which increase fracture risk include co-morbid conditions such as diabetes, obesity and chronic inflammation [59]. Physical activity which promotes mechanical strain on bone regulates the complex interplay between bone formation and remodelling, promoting enhanced bone mass [60]. Additionally, dietary supplementation with calcium and vitamin D are an integral component of osteoporosis prevention [61]. Specific recommendations regarding lifestyle modifications with effects on bone health remain undefined among women of later reproductive years.
Comparison with existing literature
Lifestyle modification interventions which target multiple potential risk factors appear to be the most effective. For example, The Diabetic Prevention Program sets specific goals for participants to achieve moderate weight loss and increases in physical activity, which has resulted in a 58% reduction in diabetes incidence [62]. Similarly, interventions combining alterations in diet and physical activity appeared to be most effective in targeting postpartum weight retention and promoting weight loss [63, 64]. Additionally, a composite healthy lifestyle score which incorporates domains relating to diet, physical activity, alcohol consumption, smoking and healthy BMI has demonstrated beneficial effects on systemic inflammation [65], pro-atherogenic lipoprotein profile [66], and childhood adiposity [67], highlighting the importance of healthy lifestyle promotion across multiple potential risk factors and stages of life.
Of late, the importance of pregnancy complications and their relationship to later life women’s health has gained increasing recognition [7, 68]. It has been suggested that women who experience GDM in pregnancy are followed prospectively in order to enable preventive measures and promote early diagnosis of type 2 diabetes mellitus, before onset of complications [68]. However, studies suggest that women who developed GDM rarely follow recommended dietary advice and physical activity guidelines in the postpartum period [69], despite evidence that modest weight loss in the postpartum period has beneficial effects on glucose homeostasis [70]. It has been suggested that increased education focusing on awareness of the magnitude and risk of later life diabetes development after GDM could provide an opportunity to introduce dietary, lifestyle, or pharmacological interventions which may prevent or delay onset of type 2 diabetes mellitus [71]. Overall, multiple interventions which include modifications to diet, promotion of weight loss, and increased physical activity appear to be effective in decreasing risks of maternal morbidity following pregnancy complications [68]. Similarly, in this scoping review, interventions which focused on physical activity and health education and promotion incorporating combined aspects of diet and physical activity modifications demonstrated beneficial effects on body composition and cardiometabolic risk factors among women in their later reproductive years.
Additionally, the importance of the post-menopausal years on women’s cardiometabolic and bone health has been previously evaluated in systematic reviews and clinical trials. Interventions focusing on diet and physical activity among postmenopausal women have demonstrated clear improvements in cardiovascular outcomes with physical activity [72, 73]. Exercise has been shown to be beneficial to maintaining balance, coordination, flexibility and muscle strength, but with conflicting evidence relating to bone health and bone mineral density [72]. No specific recommendations regarding diet have been noted [72, 73]. One intervention incorporating aspects of physical activity, health education, and behavioural change demonstrated effects on diet, exercise and motivation in post-menopausal women, which was effective in improving bone health and bone mineral density [74]. Similarly, we noted that interventions focusing on physical activity in the later reproductive years were effective in ameliorating cardiometabolic outcomes and body composition among peri-menopausal women, highlighting the possibility of earlier intervention for promotion of lifestyle modifications. Additionally, we also similarly noted no interventions focusing solely on diet recommendations, and a paucity of literature relating to bone health and bone mineral density in women of later reproductive years.
The current World Health Organization (WHO) physical activity recommendations among adults aged 18–64 is at least 150–300 min of moderate-to-vigorous aerobic activity throughout the week, in combination with muscle-strengthening activity on two or more days per week [75]. The WHO suggests 1 in 4 adults do not meet global recommended levels of physical activity [76]. In this review, improvements in cardiometabolic risk factors and body composition were noted following interventions that incorporated an element of aerobic activity and/or resistance training. Unfortunately due to wide variation in the types of exercise examined, a meta-synthesis was not achievable, and no clear consensus on the best method of aerobic or strength training, duration or intensity could be reached.
The Department of Health have published national dietary recommendations for Irish women across the lifespan, focusing on those aged 19–50 years and aged over 50 years. The recommendations for women aged 19–50 include recommendations for meeting calcium and vitamin D requirements through diet for bone health, and promotion of healthy weight during the child-bearing years. Once women are over age 50, the recommendations change to promote dietary patterns for a healthy heart, and increased intake of calcium and vitamin D through supplementation if dietary intake is insufficient, in efforts to protect against bone loss [77]. In this scoping review, there were no studies that exclusively investigated dietary patterns or calcium supplementation. A small number of studies evaluated the use of vitamin D, with equivocal outcomes, likely owing to small sample sizes and short duration of study.
While most research has focused on either the child-bearing years or post-menopausal years, the importance of the puerperium has gained recognition. The influence of body composition and implications of obesity are central in mitigating the rising incidence of NCDs [1], with postpartum weight retention gaining recognition as a potential modifiable risk factor [64]. Unfortunately, the majority of women receive little education regarding the importance of weight loss or physical activity after childbearing, highlighting the postpartum period as a potential missed opportunity for lifestyle modification [78]. Systematic reviews evaluating postpartum weight retention highlight the early postpartum years when women are still engaging heavily with health services for their children as an ideal time to initiate lifestyle modifications incorporating aspects of diet, physical activity and individualised support, in order to promote healthy weight and target postpartum weight retention [64]. While not related directly to the postpartum period, this scoping review highlighted a number of publications related to the “Women’s Healthy Lifestyle Project”, an intervention designed to promote weight control through alterations in consumption of dietary fat and cholesterol, and increase in purposeful lifestyle activity over 54 months [23–26]. The intervention was provided by allied healthcare professionals who met with participants weekly or bi-weekly for 20 weeks and provided ongoing support throughout the 54 month maintenance period [23–26]. While this was a labour-intensive intervention, this collection of publications demonstrated it was possible to promote lifestyle modifications in perimenopausal women that would prevent long-term weight gain and increases in serum cholesterol levels during the menopausal transition, another highly influential time in a woman’s life [23–26]. Acknowledging the beneficial effect of the “Women’s Healthy Lifestyle Project”, but also its intensive protocol, the “40-something RCT”, adopted a similar intervention approach with a less intensive demand on healthcare professionals, which could be implemented at a population level [79]. Motivational interviewing techniques during regular consultations with healthcare professionals, was implemented to promote weight control over 24 months during the menopausal transition. The publications resulting from this RCT included easy to promote public health messages, encouraging premenopausal women to take 10,000 steps per day and eat 5 servings of vegetables daily to prevent weight gain [20]. During the maintenance phase, women who engaged in self-directed lifestyle changes gained back the weight they had lost during the 3 month intervention, whereas ongoing support from healthcare professionals allowed weight loss to be maintained [21]. It has been suggested that interventions targeting lifestyle modifications provided by health professionals which utilize evidence-based behaviour change techniques to motivate and support participants are better equipped to support women with their overall nutrition and health [80]. While both of these interventions required extensive support from healthcare professionals, they both demonstrated that promoting a healthy lifestyle during the later reproductive years has the potential to improve risk factors associated with NCDs. The intensive protocol and demand on personnel may limit the ability to implement these strategies into routine clinical settings and pose a difficulty for long-term sustainability at a population level. Mobile health (mHealth) technology is increasing in prevalence, and may offer the support required for effective lifestyle modification, without increasing demand on clinicians [81]. In pregnancy, there has been an increased prevalence of mHealth technology to support healthy weight management [82]. Studies have demonstrated the ability of this technology to support behaviour change and lifestyle modifications [83], in a format which is acceptable to the user [84]. However, further investigation of the potential use of mHealth technology in other age groups, and across longer durations may be of benefit in promoting increased uptake of this technology to promote lifestyle modifications across multiple populations.
Clinical implications
This scoping review provides an overview of interventions focusing on potential lifestyle modifications which may be initiated during the later reproductive years. We propose the later reproductive years as an important time point in a woman’s life course where promoting a healthy lifestyle has the potential to reduce the burden of NCDs. Female-specific risk factors for cardiometabolic disease have the potential to be identified at an early age [6, 85], therefore lifestyle modifications for these women, and indeed women in general, have the potential to offset the risk of developing cardiometabolic disease in the future.
Strengths & limitations
The present scoping review possesses a number of strengths, namely ensuring evidence regarding interventions promoting lifestyle modifications in later reproductive age women are collated and presented together for consideration. This review included only human interventional studies, including RCTs and clinical trials, to gather the highest possible quality of evidence relating to lifestyle interventions. This scoping review was conducted in accordance with Arksey and O’Malley and the Joanna Briggs Institute, a process detailed extensively in the methods section in order to ensure the transparency and clarity of the scoping review.
Notwithstanding these strengths, a few limitations should be acknowledged. In general, scoping reviews synthesize a broad range of sources to identify gaps in the existing literature, often generating further research questions rather than answering only one. The frameworks used for this scoping review recommend searching multiple electronic databases, as well as manual searching of reference lists, which was performed [13, 15]. However, it must be acknowledged that it is not practical to search all possible avenues, and it is possible that other interventions have been explored, but not published. This scoping review was limited to studies published in English, potentially allowing a language bias. A significant proportion of studies also relied on self-reported patient outcomes, which may introduce response bias. Additionally, a large number of studies had small sample sizes, varying research design and were of short duration, therefore a significant degree of heterogeneity was present which limited the ability to perform meta-synthesis in order to evaluate intervention effectiveness and provide specific recommendations.
Future research
This scoping review highlighted a small number of citations from Western cultures, and Europe in particular, which would lend well to future intervention studies, in order to cover a broad range of cultural approaches to lifestyle modifications. Additionally, we noted no studies which investigated the role of dietary interventions alone, a topic that may prove beneficial in helping mitigate the future burden of chronic disease. We also noted a paucity of data in relation to outcomes related to bone disease and bone health, which should be prioritized in future research. While it has been noted that lifestyle modifications, namely increased physical activity and altered dietary pattern, have effects on cardiometabolic and bone health, the detailed mechanisms underlying these observations remains unclear and represent an important area for future study.
Since this scoping review highlighted a significant degree of variation among the included studies despite employing a systematic search protocol, this heterogeneity may limit the ability to compare results directly and provide generalised recommendations. The number of studies which investigated long-term maintenance following lifestyle modification was relatively small, highlighting an important area for future research in order to evaluate long-term sustainability of lifestyle modifications among later reproductive age women. Large scale clinical trials which investigate long-term adherence, as well as cost effectiveness of interventions, would be of benefit in addressing long-term feasibility of lifestyle modification. Future work may build on the present scoping review to systematically review evidence and conduct meta-analysis in larger clinical trials with more objective outcome measures in order to address heterogeneity and provide more specific recommendations.
Conclusion
The later reproductive years represents an important time-point in a woman’s life, and this scoping review is one of few to map interventions of lifestyle modifications related to cardiometabolic and bone disease in later reproductive age women. We present a narrative summary of interventions focusing on lifestyle modification in women of later reproductive age which may have the potential to modulate later-life NCDs. While this outcome appears possible, many uncertainties persist regarding the most effective approach to promote lifestyle change among this unique population of women.
Body composition and lipid profile were among the risk factors most commonly improved by the lifestyle interventions, however there was considerable variation among the interventions. It appeared that incorporation of regular aerobic physical activity, as well as health education targeting multiple risk factors were the most effective in improving body composition and lipid profile in women during the later reproductive years. Additionally, we have highlighted the later reproductive years as an important time point in a woman’s life where lifestyle modifications have the potential to modulate later non-communicable, chronic disease. While there are no consensus guidelines regarding the most effective interventions for lifestyle modifications among women of later reproductive age, the findings of this scoping review help strengthen the evidence for the benefit of lifestyle modifications. This information may help inform the development of clinical guidelines in the prevention of chronic disease in women in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to sincerely thank Diarmuid Stokes, UCD librarian, for his assistance in design of the initial search in collaboration with the research team.
Abbreviations
- BMI
Body mass index
- DASH
Dietary approaches to stop hypertension
- LDL cholesterol
Low density lipoprotein cholesterol
- MeSH
Medical subject headings
- MTCC qigong
Muscle/tendon change classic qigong
- NCDs
Non-communicable, chronic diseases
- PICO
Population, Intervention, Comparator, Outcome framework
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension for Scoping Reviews
- RCT
Randomized controlled trial
- WHO
World Health Organization
- FSH
Follicle stimulating hormone
- LH
Luteinizing hormone
- mHealth
Mobile health
Author contributions
KD designed and carried out the search, screened citations, reviewed the data and wrote the manuscript. GD screened citations, reviewed the data and reviewed the manuscript. RKC critically appraised the manuscript for intellectual content. CP critically appraised the manuscript for intellectual content. PT critically appraised the manuscript for intellectual content. FMcA conceived the idea for the review and revised and approved the manuscript. All authors read and approved the final manuscript.
Funding
Funding agencies had no role in this review.
Data availability
The full search strings for the PubMed database are available in supplementary Table 1 (Additional File 1). No new data was created in this study. Detailed summary of all interventions are available in supplementary file 2 (Additional file 2). The data extraction form is available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Noncommunicable disease [Webpage]. World Health Organization; 2023 [June 20, 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- 2.World Health Organization. Musculoskeletal health 2022 [updated 14 July 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions
- 3.Williams A, Kamper SJ, Wiggers JH, O’Brien KM, Lee H, Wolfenden L, et al. Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med. 2018;16(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and Prevention Strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–66. [DOI] [PubMed] [Google Scholar]
- 6.Young L, Cho L. Unique cardiovascular risk factors in women. Heart. 2019;105(21):1656–60. [DOI] [PubMed] [Google Scholar]
- 7.McNestry C, Killeen SL, Crowley RK, McAuliffe FM. Pregnancy complications and later life women’s health. Acta Obstet Gynecol Scand. 2023;102(5):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, et al. The absolute and relative risk of type 2 diabetes after gestational diabetes: a systematic review and meta-analysis of 129 studies. Diabetes Res Clin Pract. 2021;171:108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–14. [DOI] [PubMed] [Google Scholar]
- 10.Kaaja RJ. Metabolic syndrome and the menopause. Menopause Int. 2008;14(1):21–5. [DOI] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30. [DOI] [PubMed] [Google Scholar]
- 12.Woods NF, Mitchell ES, Coslov N, Richardson MK. Transitioning to the menopausal transition: a scoping review of research on the late reproductive stage in reproductive aging. Menopause. 2021;28(4):447–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arksey H, O’malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 14.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6. [DOI] [PubMed] [Google Scholar]
- 15.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 16.Hassan II, Muhammad IN, Ismail SB, Kadir AA, Noor NM, Nik Hussain NH. Effect of health education program on cardiovascular risk factors among menopausal women in Malaysia. Int Med J. 2017;24(1):51–5. [Google Scholar]
- 17.Cussler EC, Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, et al. Maintenance of weight loss in overweight middle-aged women through the internet. Obes (Silver Spring). 2008;16(5):1052–60. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Martin CJ, Metcalfe LL, et al. Weight loss readiness in middle-aged women: psychosocial predictors of success for behavioral weight reduction. J Behav Med. 2002;25(6):499–523. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28(9):1124–33. [DOI] [PubMed] [Google Scholar]
- 20.Hollis JL, Williams LT, Young MD, Pollard KT, Collins CE, Morgan PJ. Compliance to step count and vegetable serve recommendations mediates weight gain prevention in mid-age, premenopausal women. Findings of the 40-Something RCT. Appetite. 2014;83:33–41. [DOI] [PubMed] [Google Scholar]
- 21.Williams LT, Collins CE, Morgan PJ, Hollis JL. Maintaining the outcomes of a successful weight gain prevention intervention in mid-age women: two year results from the 40-something randomized control trial. Nutrients. 2019;11(5). [DOI] [PMC free article] [PubMed]
- 22.Williams LT, Hollis JL, Collins CE, Morgan PJ. Can a relatively low-intensity intervention by health professionals prevent weight gain in mid-age women? 12-month outcomes of the 40-something randomised controlled trial. Nutr Diabetes. 2014;4(5). [DOI] [PMC free article] [PubMed]
- 23.Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation. 2001;103(1):32–7. [DOI] [PubMed] [Google Scholar]
- 24.Simkin-Silverman L, Wing RR, Hansen DH, Klem ML, Pasagian-Macaulay AP, Meilahn EN, et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med. 1995;24(5):509–17. [DOI] [PubMed] [Google Scholar]
- 25.Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26(3):212–20. [DOI] [PubMed] [Google Scholar]
- 26.Simkin-Silverman LR, Wing RR, Boraz MA, Meilahn EN, Kuller LH. Maintenance of cardiovascular risk factor changes among middle-aged women in a lifestyle intervention trial. Womens Health. 1998;4(3):255–71. [PubMed] [Google Scholar]
- 27.Hao S, Tan S, Li J, Li W, Li J, Cai X, et al. Dietary and exercise interventions for metabolic health in perimenopausal women in Beijing. Asia Pac J Clin Nutr. 2021;30(4):624–31. [DOI] [PubMed] [Google Scholar]
- 28.Aneis YM, El Refaye GE, Taha MM, Aldhahi MI, Elsisi HF. Concurrent aerobic and strength training with caloric restriction reduces insulin resistance in obese premenopausal women: a randomized controlled trial. Med (Kaunas). 2023;59(7). [DOI] [PMC free article] [PubMed]
- 29.Chung J, Kim K, Hong J, Kong HJ. Effects of prolonged exercise versus multiple short exercise sessions on risk for metabolic syndrome and the atherogenic index in middle-aged obese women: a randomised controlled trial. BMC Womens Health. 2017;17(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karatrantou K, Gerodimos V, Häkkinen K, Zafeiridis A. Health-promoting effects of serial vs. Integrated Combined Strength and Aerobic Training. Int J Sports Med. 2017;38(1):55–64. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan S, Gustafson MB, Campbell C, Gaikwad NW, Keim NL. Association between circulating endogenous androgens and insulin sensitivity changes with exercise training in midlife women. Menopause. 2014;21(9):967–74. [DOI] [PubMed] [Google Scholar]
- 32.Oh DH, Lee JK. Effect of different intensities of aerobic Exercise combined with resistance exercise on body fat, lipid profiles, and adipokines in middle-aged women with obesity. Int J Environ Res Public Health. 2023;20(5). [DOI] [PMC free article] [PubMed]
- 33.Pilch W, Tota Ł, Sadowska-Krępa E, Piotrowska A, Kępińska M, Pałka T, et al. The Effect of a 12-Week Health Training Program on selected anthropometric and biochemical variables in middle-aged women. Biomed Res Int. 2017;2017:9569513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro MA, Martins MA, Carvalho CR. Interventions to increase physical activity in middle-age women at the workplace: a randomized controlled trial. Med Sci Sports Exerc. 2014;46(5):1008–15. [DOI] [PubMed] [Google Scholar]
- 35.Park SK, Park JH, Kwon YC, Kim HS, Yoon MS, Park HT. The effect of combined aerobic and resistance exercise training on abdominal fat in obese middle-aged women. J Physiol Anthropol Appl Hum Sci. 2003;22(3):129–35. [DOI] [PubMed] [Google Scholar]
- 36.Tsai YK, Chen HH, Lin IH, Yeh ML. Qigong improving physical status in middle-aged women. West J Nurs Res. 2008;30(8):915–27. [DOI] [PubMed] [Google Scholar]
- 37.Moslehi N, Vafa M, Rahimi-Foroushani A, Golestan B. Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. J Res Med Sci. 2012;17(7):607–14. [PMC free article] [PubMed] [Google Scholar]
- 38.Moslehi N, Vafa M, Sarrafzadeh J, Rahimi-Foroushani A. Does magnesium supplementation improve body composition and muscle strength in middle-aged overweight women? A double-blind, placebo-controlled, randomized clinical trial. Biol Trace Elem Res. 2013;153(1–3):111–8. [DOI] [PubMed] [Google Scholar]
- 39.Costa RR, Pilla C, Buttelli ACK, Barreto MF, Vieiro PA, Alberton CL, et al. Water-based aerobic training successfully improves lipid Profile of Dyslipidemic women: a Randomized Controlled Trial. Res Q Exerc Sport. 2018;89(2):173–82. [DOI] [PubMed] [Google Scholar]
- 40.Farahati S, Attarzadeh Hosseini S, Moazzami M, Daloee M, Daloee S. The impact of high-intensity interval training versus moderate-intensity continuous training on carotid intima-media thickness and ankle-brachial index in middle-aged women. Int J Preventive Med. 2020;11(1). [DOI] [PMC free article] [PubMed]
- 41.Kim MC, Park HS, Paik JK, Jung DY, Lee SM. Changes in serum lipids according to the amount of exercise activity in middle-aged women. Indian J Public Health Res Dev. 2018;9(12):2068–72. [Google Scholar]
- 42.Zhang J, Chen G, Lu W, Yan X, Zhu S, Dai Y, et al. Effects of physical exercise on health-related quality of life and blood lipids in perimenopausal women: a randomized placebo-controlled trial. Menopause. 2014;21(12):1269–76. [DOI] [PubMed] [Google Scholar]
- 43.Obaya HE, Abdeen HA, Salem AA, Shehata MA, Aldhahi MI, Muka T et al. Effect of aerobic exercise, slow deep breathing and mindfulness meditation on cortisol and glucose levels in women with type 2 diabetes mellitus: a randomized controlled trial. Front Physiol. 2023;14. [DOI] [PMC free article] [PubMed]
- 44.Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause. 1999;6(1):7–13. [PubMed] [Google Scholar]
- 45.Kheyruri F, Sarrafzadeh J, Hosseini AF, Abiri B, Vafa M. Randomized study of the effects of Vitamin D and Magnesium Co-supplementation on muscle strength and function, body composition, and inflammation in vitamin D-Deficient middle-aged women. Biol Trace Elem Res. 2021;199(7):2523–34. [DOI] [PubMed] [Google Scholar]
- 46.Salamone LM, Cauley JA, Black DM, Simkin-Silverman L, Lang W, Gregg E, et al. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr. 1999;70(1):97–103. [DOI] [PubMed] [Google Scholar]
- 47.Chinnappan SM, George A, Evans M, Anthony J. Efficacy of labisia pumila and eurycoma longifolia standardised extracts on hot flushes, quality of life, hormone and lipid profile of peri-menopausal and menopausal women: a randomised, placebo-controlled study. Food Nutr Res. 2020;64:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luzzi R, Belcaro G, Hosoi M, Feragalli B, Cornelli U, Dugall M, et al. Normalization of cardiovascular risk factors in peri-menopausal women with Pycnogenol®. Minerva Ginecol. 2017;69(1):29–34. [DOI] [PubMed] [Google Scholar]
- 49.Weichmann F, Rohdewald P. Pycnogenol((R)) French maritime pine bark extract in randomized, double-blind, placebo-controlled human clinical studies. Front Nutr. 2024;11:1389374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abiri B, Vafa MR, Dehghani M, Moslehi N, Sarrafzadeh J. Effect of vitamin D supplement consumption on muscle strength, muscle function and body composition in vitamin D-deficient middle-aged women: a Randomized Clinical Trial. Nutr Food Sci Res. 2016;3(3):17–24. [Google Scholar]
- 51.Jamshidi S, Masoumi SJ, Abiri B, Sarbakhsh P, Sarrafzadeh J, Nasimi N, et al. The effect of synbiotic and vitamin D co-supplementation on body composition and quality of life in middle-aged overweight and obese women: a randomized controlled trial. Clin Nutr ESPEN. 2022;52:270–6. [DOI] [PubMed] [Google Scholar]
- 52.Talaulikar V. Menopause transition: physiology and symptoms. Best Pract Res Clin Obstet Gynaecol. 2022;81:3–7. [DOI] [PubMed] [Google Scholar]
- 53.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas-Mitchell EJ, et al. Lipid changes around the final menstrual period predict carotid subclinical disease in Postmenopausal Women. Stroke. 2017;48(1):70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El Khoudary SR, Thurston RC. Cardiovascular implications of the menopause transition: endogenous sex hormones and vasomotor symptoms. Obstet Gynecol Clin North Am. 2018;45(4):641–61. [DOI] [PubMed] [Google Scholar]
- 56.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7). [DOI] [PMC free article] [PubMed]
- 57.Wang W, Liu Y, Li Y, Luo B, Lin Z, Chen K et al. Dietary patterns and cardiometabolic health: Clinical evidence and mechanism. MedComm (2020). 2023;4(1):e212. [DOI] [PMC free article] [PubMed]
- 58.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the study of women’s Health across the Nation (SWAN). J Bone Min Res. 2012;27(1):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karlamangla AS, Burnett-Bowie SM, Crandall CJ. Bone Health during the Menopause Transition and Beyond. Obstet Gynecol Clin North Am. 2018;45(4):695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang X, Xu S, Zhang H. Regulation of bone health through physical exercise: mechanisms and types. Front Endocrinol (Lausanne). 2022;13:1029475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;2013(7):CD005627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14(10):792–805. [DOI] [PubMed] [Google Scholar]
- 65.Millar SR, Harrington JM, Perry IJ, Phillips CM. Associations between a protective lifestyle behaviour score and biomarkers of chronic low-grade inflammation: a cross-sectional analysis in middle-to-older aged adults. Int J Obes (Lond). 2022;46(3):476–85. [DOI] [PubMed] [Google Scholar]
- 66.Millar SR, Harrington JM, Perry IJ, Phillips CM. Protective lifestyle behaviours and lipoprotein particle subclass profiles in a middle-to older-aged population. Atherosclerosis. 2020;314:18–26. [DOI] [PubMed] [Google Scholar]
- 67.Navarro P, Mehegan J, Murrin CM, Kelleher CC, Phillips CM, Lifeways Cross Generation Cohort S. Associations between a maternal healthy lifestyle score and adverse offspring birth outcomes and childhood obesity in the lifeways cross-generation Cohort Study. Int J Obes (Lond). 2020;44(11):2213–24. [DOI] [PubMed] [Google Scholar]
- 68.Neiger R. Long-term effects of pregnancy complications on maternal health: a review. J Clin Med. 2017;6(8). [DOI] [PMC free article] [PubMed]
- 69.Kaiser B, Razurel C. Determinants of postpartum physical activity, dietary habits and weight loss after gestational diabetes mellitus. J Nurs Manag. 2013;21(1):58–69. [DOI] [PubMed] [Google Scholar]
- 70.Ehrlich SF, Hedderson MM, Quesenberry CP Jr., Feng J, Brown SD, Crites Y, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med. 2014;31(7):862–7. [DOI] [PMC free article] [PubMed]
- 71.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. [DOI] [PubMed] [Google Scholar]
- 72.Pines A. Lifestyle and diet in postmenopausal women. Climacteric. 2009;12(Suppl 1):62–5. [DOI] [PubMed] [Google Scholar]
- 73.Cheng CC, Hsu CY, Liu JF. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women: a systematic review and meta-analysis. Menopause. 2018;25(7):772–82. [DOI] [PubMed] [Google Scholar]
- 74.Anupama DS, Noronha JA, Acharya KKV, Prabhu M, Ravishankar N, Nayak BS. Effect of Lifestyle Modification Intervention Programme on Bone Mineral density among postmenopausal women with osteoporosis. Sultan Qaboos Univ Med J. 2023;23(3):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.World Health Organization. Global recommendations on physical activity for health; 2010. [PubMed]
- 76.World Health Organization. Physical activity. Web-page; 2022.
- 77.Food Safety Authority of Ireland. Healthy eating, food safety and food legislation: A guide supporting the Healthy Ireland Food Pyramid. In: Ireland Department of Health, and Food Safety Authority of Ireland, editor. Ireland; 2019.
- 78.Ferrari RM, Siega-Riz AM, Evenson KR, Moos MK, Melvin CL, Herring AH. Provider advice about weight loss and physical activity in the postpartum period. J Womens Health (Larchmt). 2010;19(3):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams LT, Hollis JL, Collins CE, Morgan PJ. The 40-Something randomized controlled trial to prevent weight gain in mid-age women. BMC Public Health. 2013;13:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watson D, Jacob CM, Giles G, McAuliffe FM, Godfrey K, Hanson M. A scoping review of nutritional interventions and policy guidelines in the interconception period for prevention of noncommunicable diseases. Reproductive Female Child Health. 2022;1(1):18–41. [Google Scholar]
- 81.O’Brien OA, McCarthy M, Gibney ER, McAuliffe FM. Technology-supported dietary and lifestyle interventions in healthy pregnant women: a systematic review. Eur J Clin Nutr. 2014;68(7):760–6. [DOI] [PubMed] [Google Scholar]
- 82.Chan KL, Chen M. Effects of Social Media and Mobile Health Apps on pregnancy care: Meta-Analysis. JMIR Mhealth Uhealth. 2019;7(1):e11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ainscough KM, O’Brien EC, Lindsay KL, Kennelly MA, O’Sullivan EJ, O’Brien OA, et al. Nutrition, Behavior Change and Physical Activity outcomes from the PEARS RCT-An mHealth-Supported, lifestyle intervention among pregnant women with overweight and obesity. Front Endocrinol (Lausanne). 2019;10:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greene EM, O’Brien EC, Kennelly MA, O’Brien OA, Lindsay KL, McAuliffe FM. Acceptability of the pregnancy, Exercise, and Nutrition Research Study with Smartphone App Support (PEARS) and the Use of Mobile Health in a mixed lifestyle intervention by pregnant obese and overweight women: secondary analysis of a Randomized Controlled Trial. JMIR Mhealth Uhealth. 2021;9(5):e17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McAuliffe FM. Impact of pregnancy on long-term health: advances in postpregnancy care-An opportunity to improve long-term maternal health. Int J Gynaecol Obstet. 2023;160(Suppl 1):4–6. [DOI] [PubMed] [Google Scholar]
- 86.El Shebini SM, Moaty MIA, Fouad S, Kazem YMI, Ahmed NH, Mohamed MS, et al. Association between serum clusterin and cognitive functions in obese Egyptian women; potential effects of dietary therapy. Der Pharma Chem. 2016;8(6):205–13. [Google Scholar]
- 87.El Shebini SM, Abdel-Moaty M, Kazem YI, Ahmed NH, Fouad S, Mohamed MS, et al. Relation between obesity, cognition and serum amyloid β protein level and potential role of foeniculum vulgare in reducing weight and improving cognitive functions. J Biol Sci. 2017;17(5):202–12. [Google Scholar]
- 88.Moaty MIA, Fouad S, El Shebini SM, Kazem YMI, Ahmed NH, Mohamed MS, et al. Serum ceramide kinase as a biomarker of cognitive functions, and the effect of using two slimming dietary therapies in obese middle aged females. Open Access Macedonian J Med Sci. 2015;3(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beak SG, Kim DJ. Effects of combined exercise on inflammatory factors and nitric oxide in obese middle-aged women. Indian J Public Health Res Dev. 2018;9(9):1266–70. [Google Scholar]
- 90.Moaty MIA, Fouad S, El Shebini SM, Kazem YI, Tapozada ST. Biochemical assessment of bone health in working obese Egyptian females with metabolic syndrome; the effect of weight loss by natural dietary therapies. Open Access Macedonian J Med Sci. 2015;3(4):582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mortimer J, McKune AJ. Effect of short-term isometric handgrip training on blood pressure in middle-aged females. Cardiovasc J Afr. 2011;22(5):257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full search strings for the PubMed database are available in supplementary Table 1 (Additional File 1). No new data was created in this study. Detailed summary of all interventions are available in supplementary file 2 (Additional file 2). The data extraction form is available from the corresponding author upon reasonable request.