Abstract
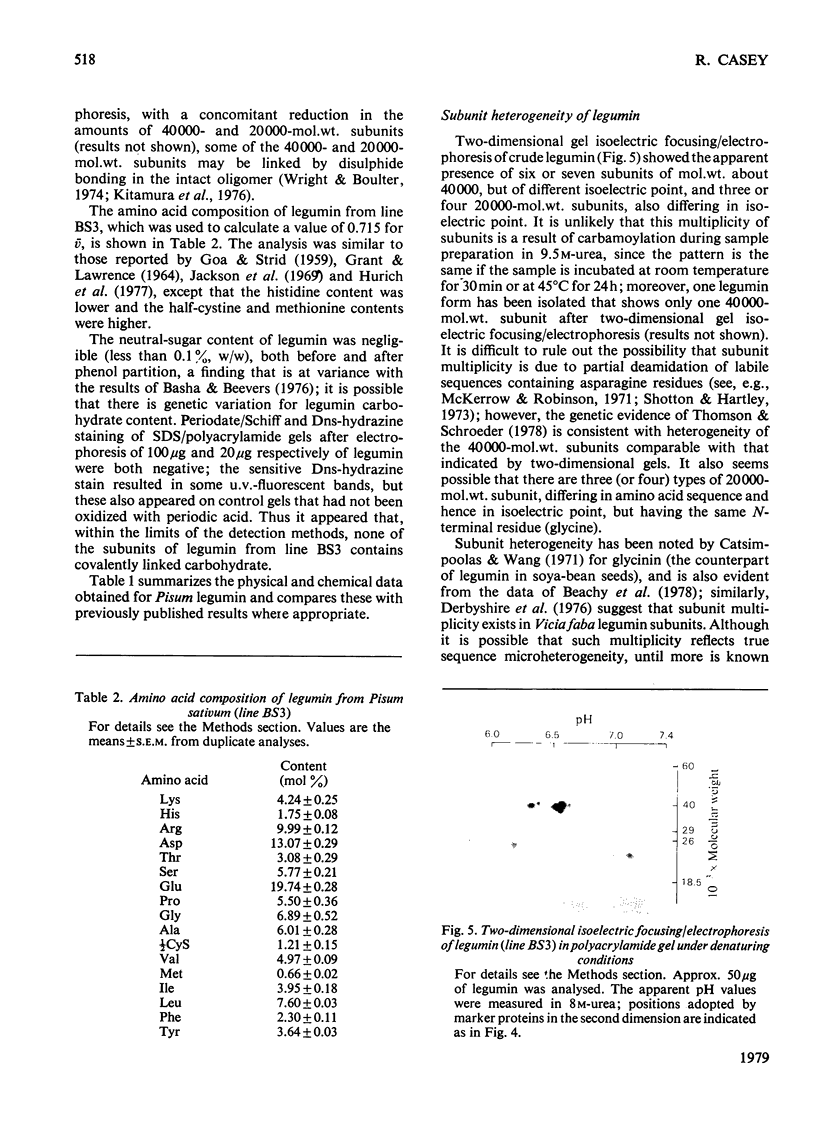
The potential of immunoaffinity chromatography as a means of purifying legumin from a wide range of Pisum (pea) types was assessed. The method required small amounts of highly purified legumin from a single Pisum type, and this was obtained by salting out with (NH4)2SO4 followed by zonal isoelectric precipitation, ion-exchange chromatography on DEAE-cellulose and sucrose-density-gradient centrifugation. Some physiocochemical properties of purified legumin were determined, a number of which (Strokes radius, subunit molecular weights, subunit N-terminal residues and subunit molar ratios) have not previously been reported for Pisum legumin. Examination of Pisum legumin by two-dimensional gel isoelectric focusing/electrophoresis indicated the existence of extensive subunit heterogeneity, and polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate showed apparent variation in the nature of this heterogeneity from one Pisum variety to another. Despite this variation, immunoaffinity chromatography on immobilized anti-legumin (which was prepared by affinity chromatography on the immubolized purified legumin from the single Pisum type) was shown to be a generally applicable method for the purification of undegraded legumin from a range of pisum types, including two primate lines.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Willis D. D., Holladay D. W., Caton J. E., Holleman J. W., Eveleigh J. W., Attrill J. E., Ball F. L., Anderson N. L. Analytical techniques for cell fractions. XX. Cyclic affinity chromatography: principles and applications. Anal Biochem. 1975 Oct;68(2):371–393. doi: 10.1016/0003-2697(75)90634-x. [DOI] [PubMed] [Google Scholar]
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Catsimpoolas N., Wang J. Analytical scanning isoelectrofocusing. 5. Separation of glycinin subunits in urea-dithiothreitol media. Anal Biochem. 1971 Dec;44(2):436–444. doi: 10.1016/0003-2697(71)90230-2. [DOI] [PubMed] [Google Scholar]
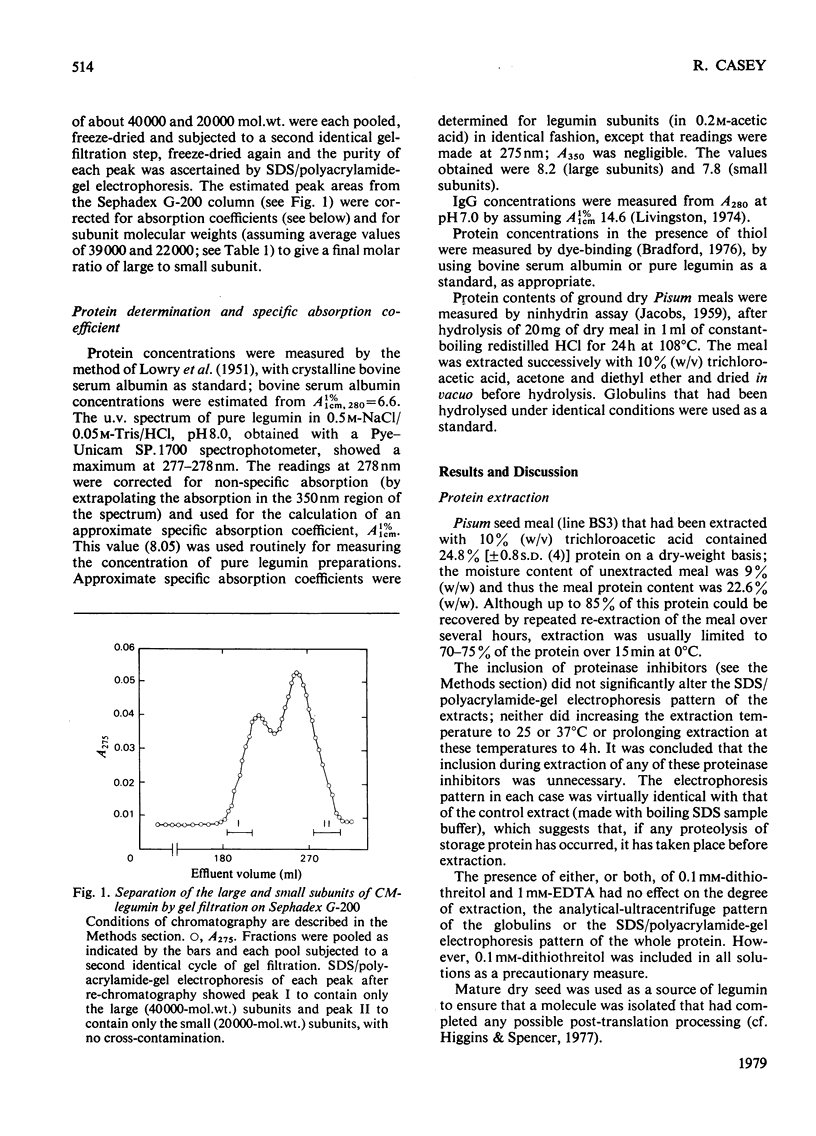
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt A. E., Hayes C. E., Goldstein I. J. A sensitive fluorescent method for the detection of glycoproteins in polyacrylamide gels. Anal Biochem. 1976 May 21;73(1):192–197. doi: 10.1016/0003-2697(76)90154-8. [DOI] [PubMed] [Google Scholar]
- GOA J., STRID L. Amino acid content of leguminous proteins as affected by genetic and nutritional factors III. Arch Mikrobiol. 1959;33:253–259. doi: 10.1007/BF00409799. [DOI] [PubMed] [Google Scholar]
- GRANT D. R., LAWRENCE J. M. EFFECTS OF SODIUM DODECYL SULFATE AND OTHER DISSOCIATING REAGENTS ON THE GLOBULINS OF PEAS. Arch Biochem Biophys. 1964 Dec;108:552–561. doi: 10.1016/0003-9861(64)90441-2. [DOI] [PubMed] [Google Scholar]
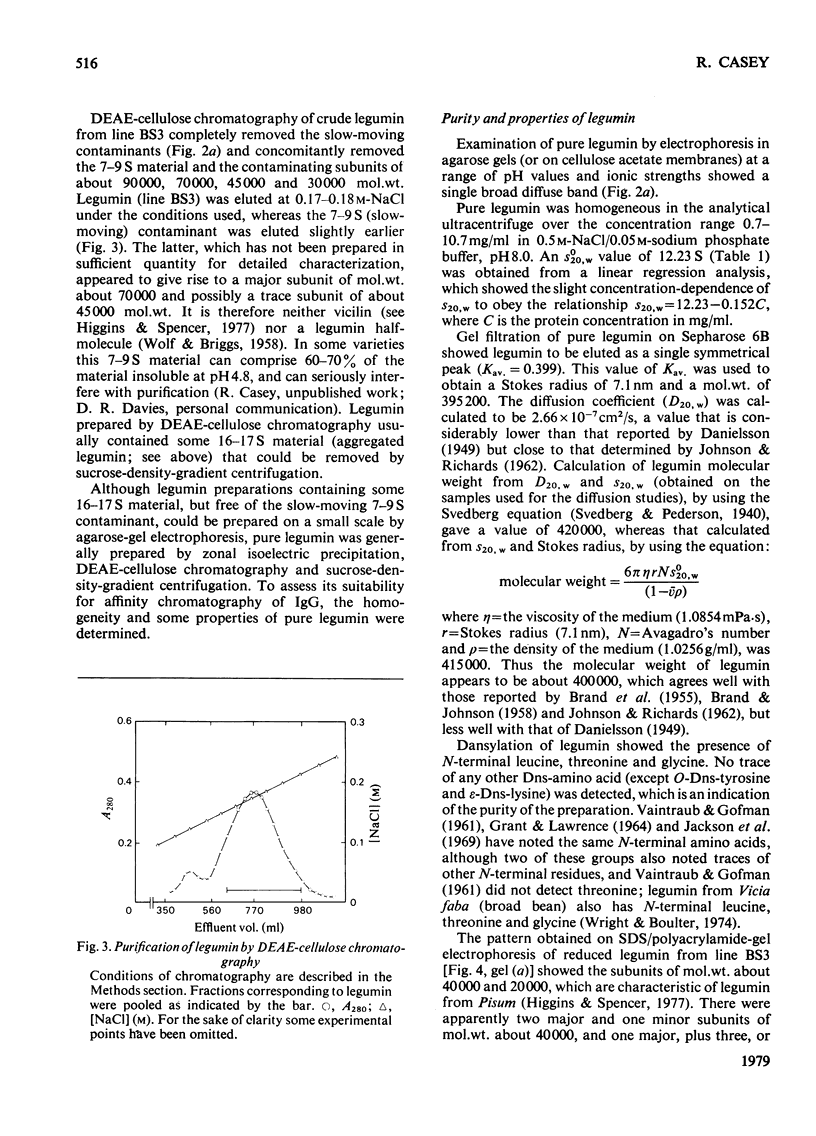
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
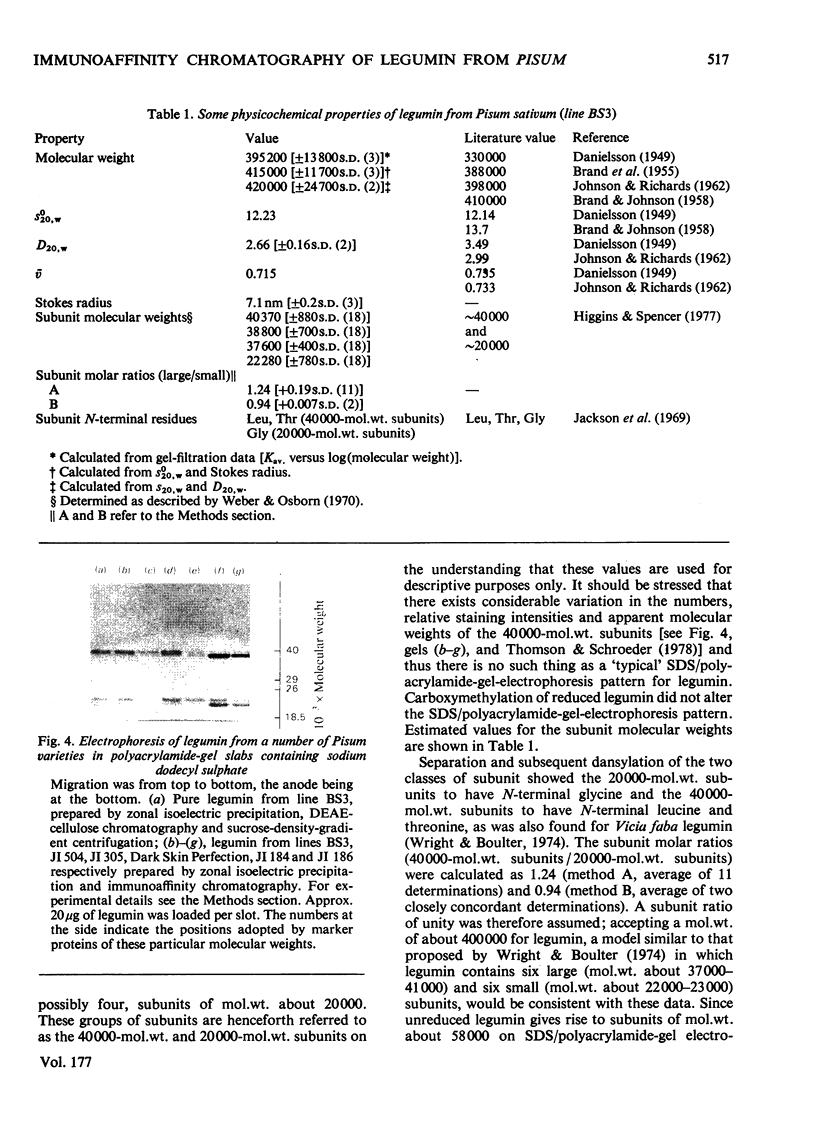
- Higgins T. J., Spencer D. Cell-free Synthesis of Pea Seed Proteins. Plant Physiol. 1977 Nov;60(5):655–661. doi: 10.1104/pp.60.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS S. Determination of nitrogen in proteins by means of idanetrione hydrate. Nature. 1959 Jan 24;183(4656):262–262. doi: 10.1038/183262a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., RICHARDS E. G. The study of legumin by depolarization of fluorescence and other physicochemical methods. Arch Biochem Biophys. 1962 May;97:260–276. doi: 10.1016/0003-9861(62)90078-4. [DOI] [PubMed] [Google Scholar]
- Kerckaert J. P., Bayard B., Debray H., Sautière P., Biserte G. Rat alpha-fetoprotein heterogeneity. Comparative chemical study of the two electrophoretic variants and their Ricinus lectin-binding properties. Biochim Biophys Acta. 1977 Aug 23;493(2):293–303. doi: 10.1016/0005-2795(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Koshiyama I. Purification and physico-chemical properties of 11S globulin in soybean seeds. Int J Pept Protein Res. 1972;4(3):167–176. doi: 10.1111/j.1399-3011.1972.tb03416.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Lås T. Improved agarose for immunoelectrophoresis. Anal Biochem. 1976 May 7;72:527–532. doi: 10.1016/0003-2697(76)90562-5. [DOI] [PubMed] [Google Scholar]
- MARKHAM R. A graphical method for the rapid determination of sedimentation coefficients. Biochem J. 1960 Dec;77:516–519. doi: 10.1042/bj0770516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H., Robinson A. B. Deamidation of asparaginyl residues as a hazard in experimental protein and peptide procedures. Anal Biochem. 1971 Aug;42(2):565–568. doi: 10.1016/0003-2697(71)90074-1. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Leicht W., Werber M. M. Hydrophobic chromatography and fractionation of enzymes from extremely halophilic bacteria using decreasing concentration gradients of ammonium sulfate. Biochemistry. 1976 Jun 1;15(11):2383–2387. doi: 10.1021/bi00656a021. [DOI] [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. Interactions of proteins with other polyelectrolytes in a two-phase system containing phenol and aqueous buffers at various pH values. Biochem J. 1966 Apr;99(1):93–101. doi: 10.1042/bj0990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. W. The estimation of threonine and serine in proteins. Biochem J. 1946;40(5-6):632–640. doi: 10.1042/bj0400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Evidence for the amino acid sequence of porcine pancreatic elastase. Biochem J. 1973 Apr;131(4):643–675. doi: 10.1042/bj1310643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutov A. D., Vaintraub I. A. Prymenenye metoda zonnoho osazhdenyia dlia razdelenyia hlobulynov semian bobovykh. Ukr Biokhim Zh. 1965;37(2):177–181. [PubMed] [Google Scholar]
- VAINTRAUB Ia, GOFMAN Iu Ia. [N-terminal amino acids in pea legumin and vicilin]. Biokhimiia. 1961 Jan-Feb;26:13–17. [PubMed] [Google Scholar]
- WOLF W. J., BRIGGS D. R. Studies on the cold-insoluble fraction of the water-extractable soybean proteins. II. Factors influencing conformation changes in the 11 S component. Arch Biochem Biophys. 1958 Aug;76(2):377–393. doi: 10.1016/0003-9861(58)90163-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Wright D. J., Boulter D. Purification and subunit structure of legumin of Vicia faba L. (broad bean). Biochem J. 1974 Aug;141(2):413–418. doi: 10.1042/bj1410413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Haar F. Purification of proteins by fractional interfacial salting out on unsubstituted agarose gels. Biochem Biophys Res Commun. 1976 Jun 7;70(3):1009–1013. doi: 10.1016/0006-291x(76)90692-6. [DOI] [PubMed] [Google Scholar]