Abstract
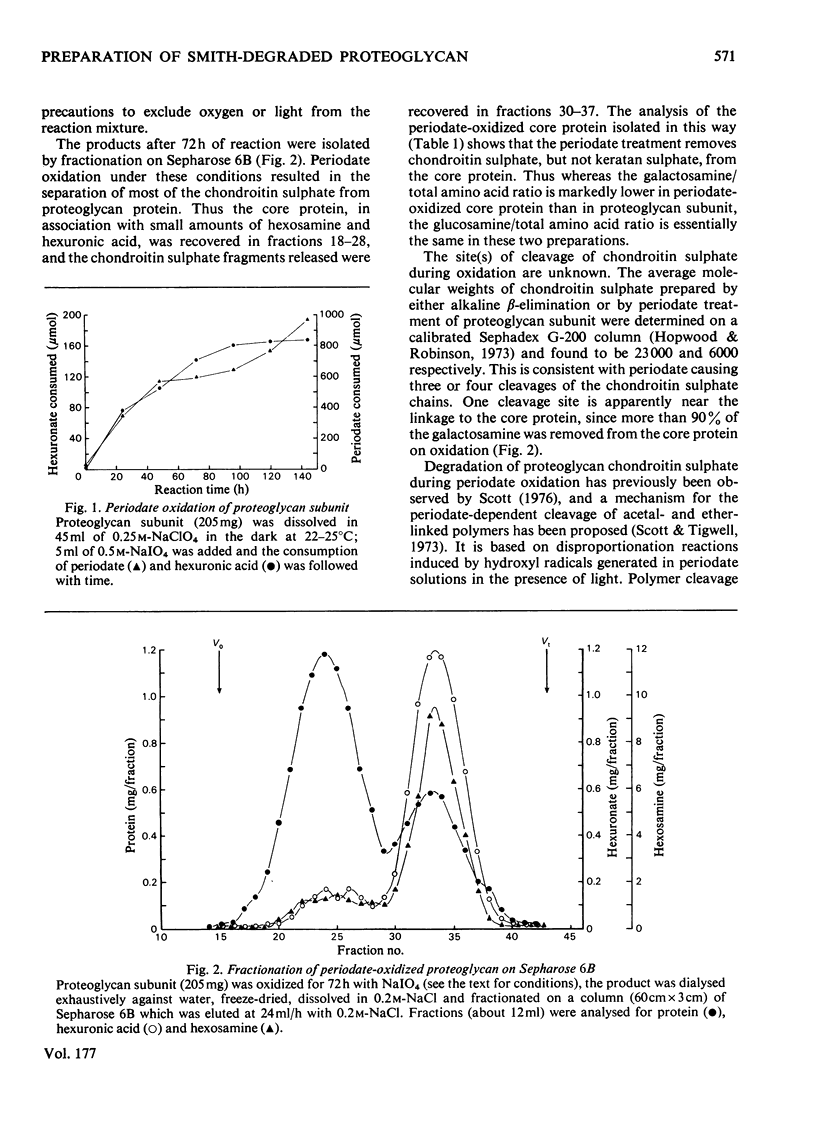
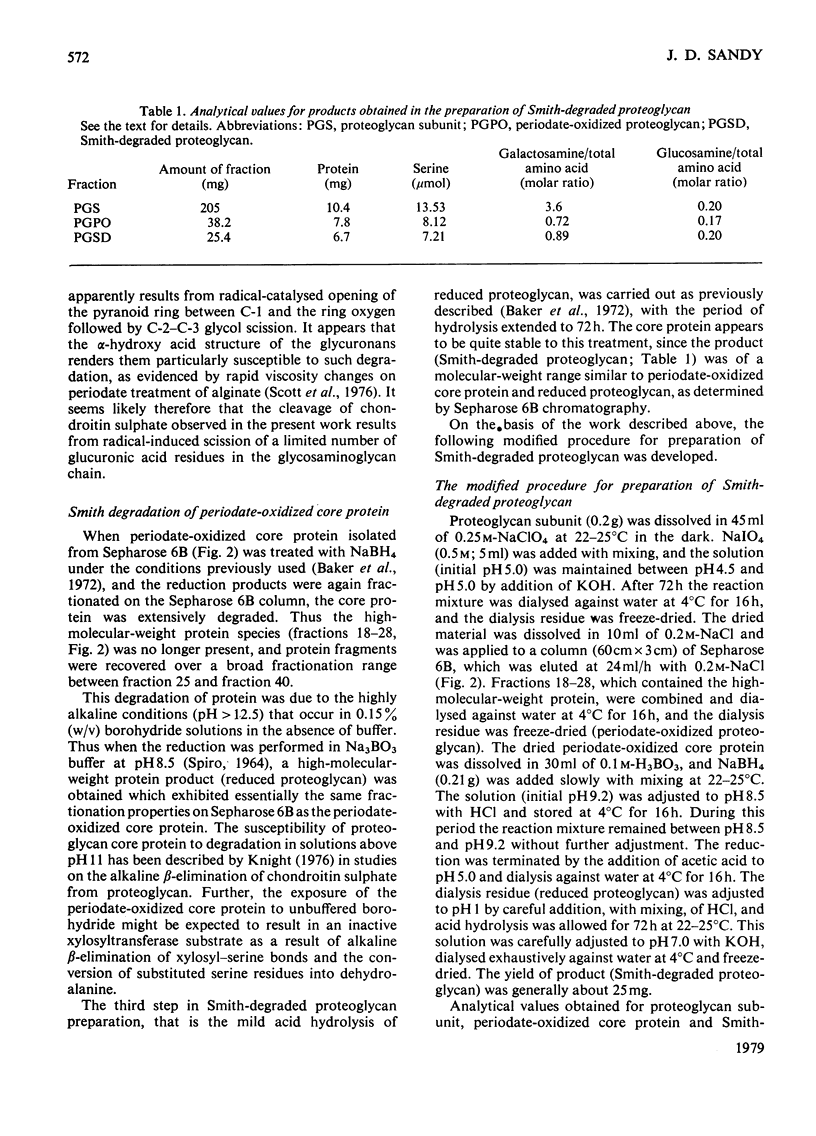
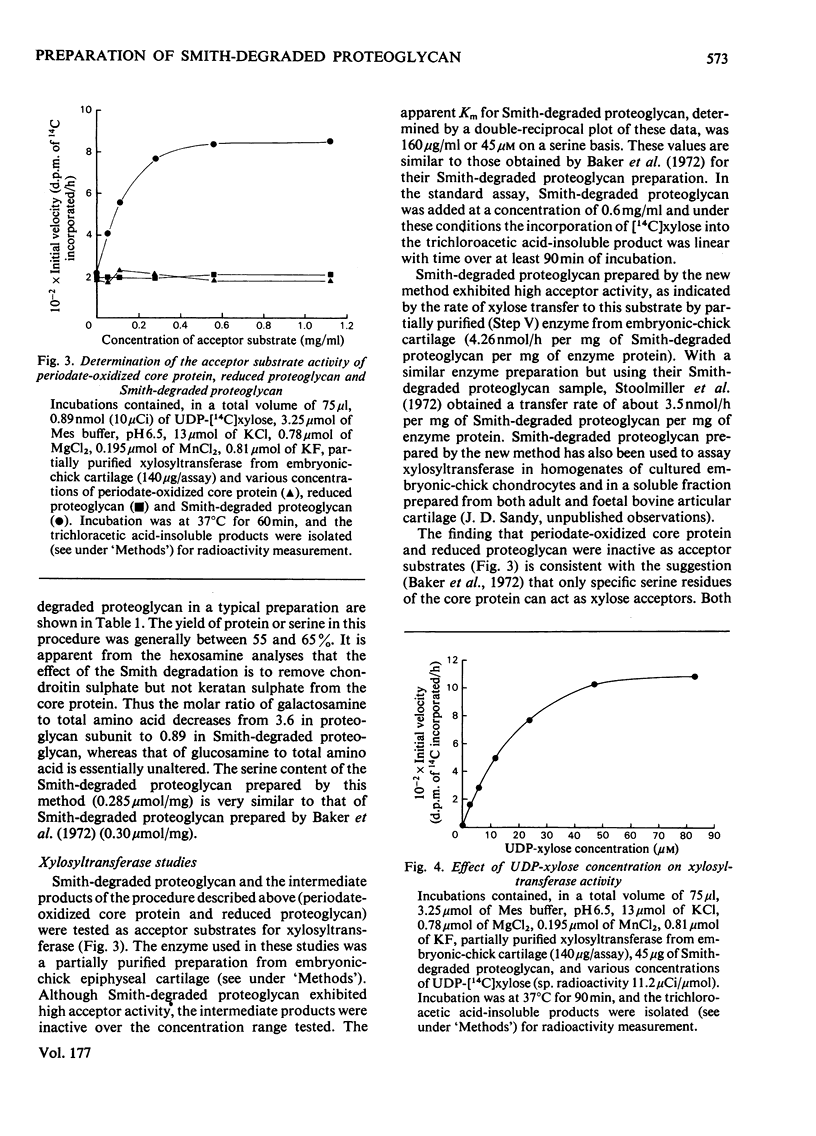
A modification of the published method [Baker, Rodén & Stoolmiller (1972) J. Biol. Chem. 247, 3838--3847] for preparation of Smith-degraded proteoglycan is described. The new method is based on the finding that most of the chondroitin sulphate is cleaved from proteoglycan core protein by periodate oxidation. The borohydride reduction procedure was modified because the periodate-oxidized core protein is extensively degraded under the highly alkaline conditions previously used. The new method involves the separation of periodate-oxidized core protein from chondroitin sulphate by gel filtration on Sepharose 6B, and the reduction of the former in H3BO3/NaBH4 at pH 8.5 to produce the reduced species. Smith-degraded proteoglycan prepared by this method exhibited high acceptor activity for xylosyltransferase from embryonic-chick cartilage and had an apparent Km of 160 microgram/ml or 45 micrometer on a serine basis. In this assay system an apparent Km of 19 micrometer was obtained for UDP-xylose. The intermediate products periodate-oxidized core protein and reduced proteoglycen were inactive as xylosyltransferase acceptor substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BOBBITT J. M. Periodate oxidation of carbohydrates. Adv Carbohydr Chem. 1956;48(11):1–41. doi: 10.1016/s0096-5332(08)60115-0. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Rodén L., Stoolmiller A. C. Biosynthesis of chondroitin sulfate proteoglycan. Xylosyl transfer to Smith-degraded cartilage proteoglycan and other exogenous acceptors. J Biol Chem. 1972 Jun 25;247(12):3838–3847. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Robinson H. C., Telser A., Dorfman A. Studies on biosynthesis of the linkage region of chondroitin sulfate-protein complex. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1859–1866. doi: 10.1073/pnas.56.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Schwartz N. B., Dorfman A. Purification of rat chondrosarcoma xylosyltransferase. Arch Biochem Biophys. 1975 Nov;171(1):136–144. doi: 10.1016/0003-9861(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Stoolmiller A. C., Horwitz A. L., Dorfman A. Biosynthesis of the chondroitin sulfate proteoglycan. Purification and properties of xylosyltransferase. J Biol Chem. 1972 Jun 10;247(11):3525–3532. [PubMed] [Google Scholar]


