Abstract
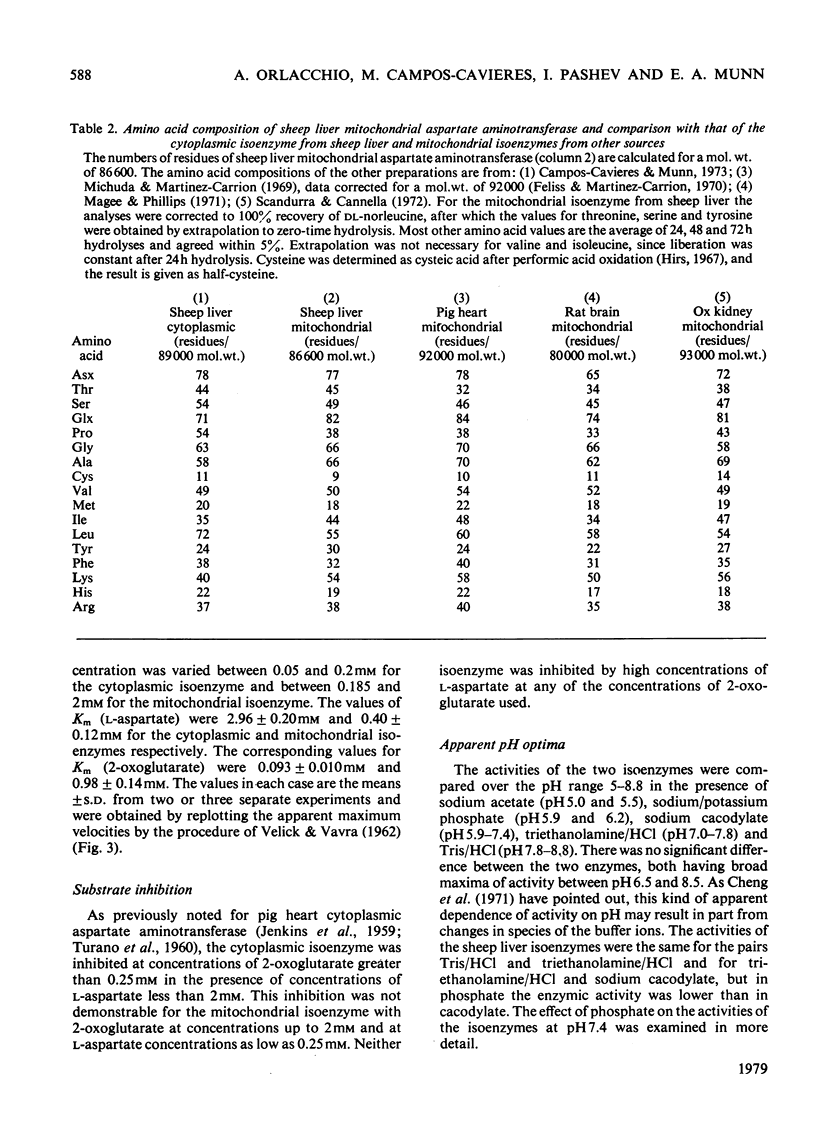
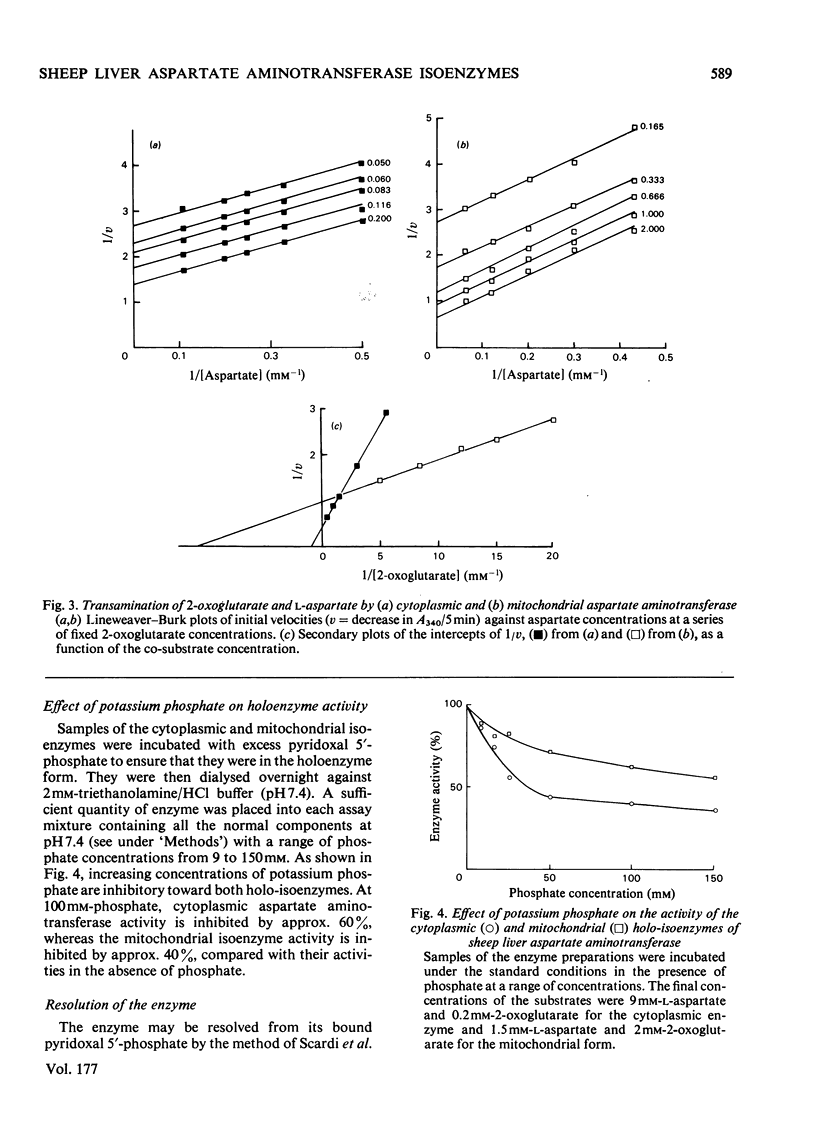
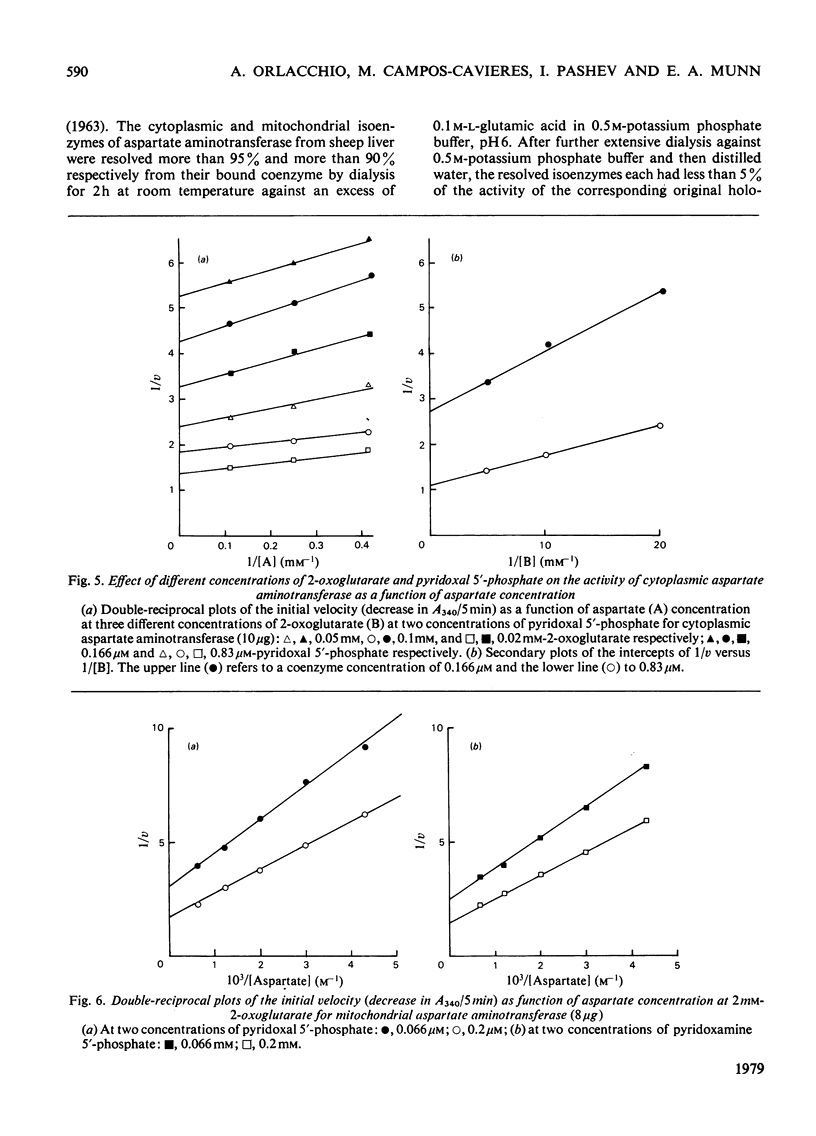
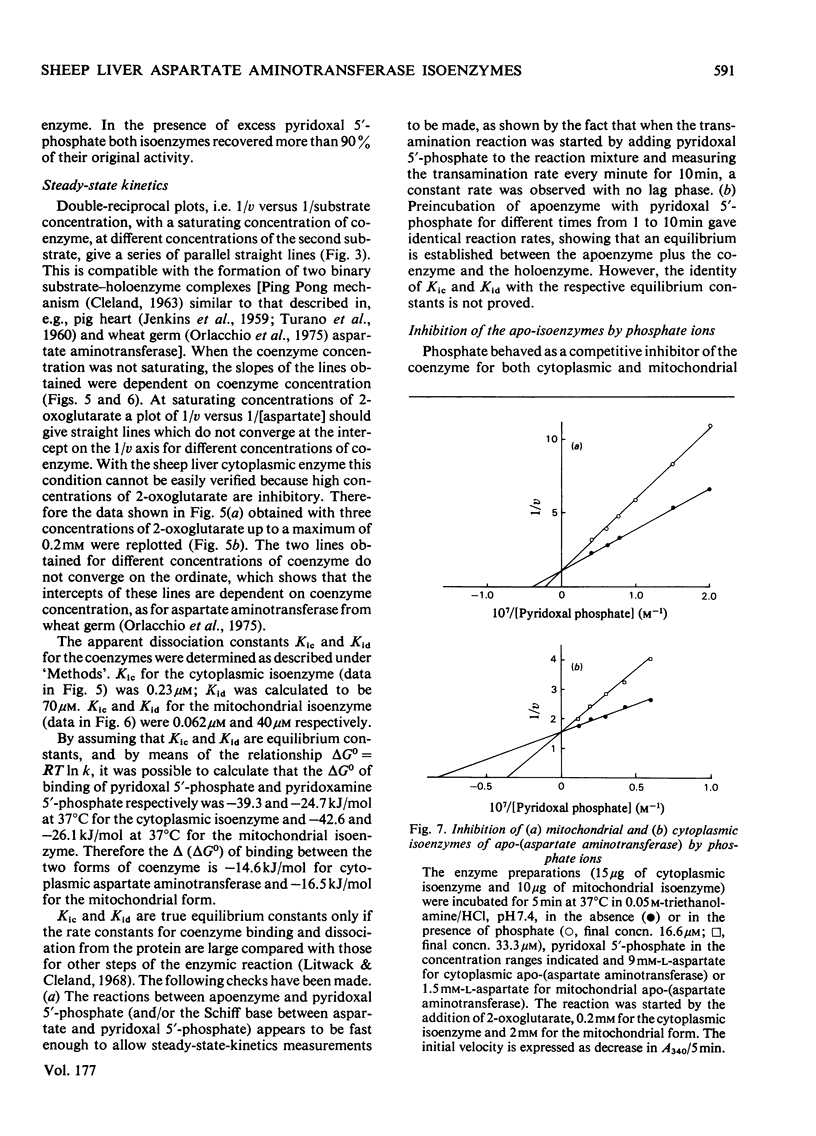
A method for the purification of mitochondrial isoenzyme of sheep liver aspartate aminotransferase (EC 2.6.1.1) is described. The final preparation is homogeneous by ultracentrifuge analyses and polyacrylamide-gel electrophoresis and has a high specific activity (182 units/mg). The molecular weight determined by sedimentation equilibrium is 87,100 +/- 680. The amino acid composition is presented; it is similar to that of other mitochondrial isoenzymes, but with a higher content of tyrosine and threonine. Subforms have been detected. On isoelectric focusing a broad band was obtained, with pI 9.14. The properties of the mitochondrial aspartate aminotransferase are compared with those of the cytoplasmic isoenzyme. The Km for L-aspartate and 2-oxoglutarate for the cytoplasmic enzyme were 2.96 +/- 0.20 mM and 0.093 +/- 0.010 mM respectively; the corresponding values for the mitochondrial form were 0.40 +/- 0.12 mM and 0.98 +/- 0.14 mM. Cytoplasmic aspartate aminotransferase showed substrate inhibition by concentrations of 2-oxoglutarate above 0.25 mM in the presence of aspartate up to 2mM. The mitochondrial isoenzyme was not inhibited in this way. Pi at pH 7.4 inhibited cytoplasmic holoenzyme activity by up to about 60% and mitochondrial holoenzyme activity up to 40%. The apparent dissociation constants for pyridoxal 5'-phosphate were 0.23 micrometer (cytoplasmic) and 0.062 micrometer (mitochondrial) and for pyridoxamine 5'-phosphate they were 70 micrometer (cytoplasmic) and 40 micrometer (mitochondrial). Pi competitively inhibited coenzyme binding to the apoenzymes; the inhibition constants at 37 degree C were 32 micrometer for the cytoplasmic isoenzyme and 19.5 micrometer for the mitochondrial form.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki K., Calva E., Del Alba Fonseca L. Crystallization and immunological identification of dog heart aspartate aminotransferase isoenzymes. J Biochem. 1972 Sep;72(3):511–519. doi: 10.1093/oxfordjournals.jbchem.a129930. [DOI] [PubMed] [Google Scholar]
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. W. The extraction and purification of two isoenzymes of L-aspartate:2-oxoglutarate aminotransferase. Biochim Biophys Acta. 1966 Feb 14;113(2):302–311. doi: 10.1016/s0926-6593(66)80069-3. [DOI] [PubMed] [Google Scholar]
- Boyde T. R. Aspartate aminotransferase. The effects of ionic concentration of kinetic constants of both isoenzymes. Biochem J. 1968 Feb;106(3):581–586. doi: 10.1042/bj1060581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Campos-Cavieres M., Milstein C. P. The sequences of the coenzyme-binding peptide in the cytoplasmic and the mitochondrial aspartate aminotransferases from sheep liver. Biochem J. 1975 May;147(2):275–281. doi: 10.1042/bj1470275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Cavieres M., Munn E. A. Purification and some properties of cytoplasmic aspartate aminotransferase from sheep liver. Biochem J. 1973 Dec;135(4):683–693. doi: 10.1042/bj1350683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- Cheng S., Michuda-Kozak C., Martinez-Carrion M. Effects of anions on the substrate affinities of the pyridoxal and pyridoxamine forms of mitochondrial and supernatant aspartate transaminases. J Biol Chem. 1971 Jun 10;246(11):3623–3630. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DECKER L. E., RAU E. M. Multiple forms of glutamic-oxalacetic transaminase in tissues. Proc Soc Exp Biol Med. 1963 Jan;112:144–149. doi: 10.3181/00379727-112-27975. [DOI] [PubMed] [Google Scholar]
- Doonan S., Hughes G. J., Barra D., Bossa F., Martini F., Petruzzelli R. Homology of the primary structures of cytoplasmic and mitochondrial aspartate aminotransferases from pig heart. FEBS Lett. 1974 Dec 1;49(1):25–28. doi: 10.1016/0014-5793(74)80623-x. [DOI] [PubMed] [Google Scholar]
- FLEISHER G. A., POTTER C. S., WAKIM K. G. Separation of 2 glutamic-oxalacetic transaminases by paper electrophoresis. Proc Soc Exp Biol Med. 1960 Jan;103:229–231. doi: 10.3181/00379727-103-25469. [DOI] [PubMed] [Google Scholar]
- Feliss N., Martinez-Carrion M. The molecular weight and subunits of the isozymes of glutamic aspartic transaminase. Biochem Biophys Res Commun. 1970 Aug 24;40(4):932–940. doi: 10.1016/0006-291x(70)90993-9. [DOI] [PubMed] [Google Scholar]
- HOOK R. H., VESTLING C. S. The two forms of rat-liver aspartate transaminase. Biochim Biophys Acta. 1962 Dec 4;65:358–359. doi: 10.1016/0006-3002(62)91058-2. [DOI] [PubMed] [Google Scholar]
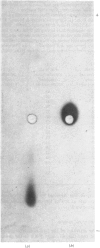
- Hopkinson D. A., Corney G., Cook P. J., Robson E. B., Harris H. Genetically determined electrophoretic variants of human red cell NADH diaphorase. Ann Hum Genet. 1970 Jul;34(1):1–10. doi: 10.1111/j.1469-1809.1970.tb00214.x. [DOI] [PubMed] [Google Scholar]
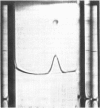
- JENKINS W. T., YPHANTIS D. A., SIZER I. W. Glutamic aspartic transaminase. I. Assay, purification, and general properties. J Biol Chem. 1959 Jan;234(1):51–57. [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- KRISTJANSSON F. K. GENETIC CONTROL OF TWO PRE-ALBUMINS IN PIGS. Genetics. 1963 Aug;48:1059–1063. doi: 10.1093/genetics/48.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N., Okada M., Nishii Y. Regulation of the urea cycle and TCA cycle by ammonia. Adv Enzyme Regul. 1966;4:317–336. doi: 10.1016/0065-2571(66)90025-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K. F., Williamson J. R. Interrelationships between malate-aspartate shuttle and citric acid cycle in rat heart mitochondria. Metabolism. 1971 Feb;20(2):119–140. doi: 10.1016/0026-0495(71)90087-4. [DOI] [PubMed] [Google Scholar]
- Litwack G., Cleland W. W. Studies on the tyrosine aminotransferase mechanism. Biochemistry. 1968 Jun;7(6):2072–2079. doi: 10.1021/bi00846a008. [DOI] [PubMed] [Google Scholar]
- MEISTER A., SOBER H. A., PETERSON E. A. Studies on the coenzyme activation of glutamic-aspartic apotransaminase. J Biol Chem. 1954 Jan;206(1):89–100. [PubMed] [Google Scholar]
- Magee S. C., Phillips A. T. Molecular properties of the multiple aspartate aminotransferases purified from rat brain. Biochemistry. 1971 Aug 31;10(18):3397–3405. doi: 10.1021/bi00794a013. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Tiemeier D. Mitochondrial glutamate-aspartate transaminase. I. Structural comparison with the supernatant isozyme. Biochemistry. 1967 Jun;6(6):1715–1722. doi: 10.1021/bi00858a021. [DOI] [PubMed] [Google Scholar]
- Michuda C. M., Martinez-Carrion M. Mitochondrial aspartate transaminase. II. Isolation and characterization of the multiple forms. Biochemistry. 1969 Mar;8(3):1095–1105. doi: 10.1021/bi00831a041. [DOI] [PubMed] [Google Scholar]
- Morino Y., Watanabe T. Primary structure of pyridoxal phosphate binding site in the mitochondrial and extramitochondrial aspartate aminotransferases from pig heart muscle. Chymotryptic peptides. Biochemistry. 1969 Aug;8(8):3412–3417. doi: 10.1021/bi00836a041. [DOI] [PubMed] [Google Scholar]
- NISSELBAUM J. S., BODANSKY O. IMMUNOCHEMICAL AND KINETIC PROPERTIES OF ANIONIC AND CATIONIC GLUTAMIC-OXALOACETIC TRANSAMINASES SEPARATED FROM HUMAN HEART AND HUMAN LIVER. J Biol Chem. 1964 Dec;239:4232–4236. [PubMed] [Google Scholar]
- Nisselbaum J. S., Bodansky O. Kinetics and electrophoretic properties of the isozymes of aspartate aminotransferase from pig heart. J Biol Chem. 1966 Jun 10;241(11):2661–2664. [PubMed] [Google Scholar]
- Orlacchio A., Scaramuzza E., Turano C. Aspartate aminotransferase from wheat germ: purification and kinetic properties. Ital J Biochem. 1975 Mar-Apr;24(2):119–137. [PubMed] [Google Scholar]
- SCARDI V., SCOTTO P., IACCARINO M., SCARANO E. The binding of pyridoxal 5-phosphate to aspartate aminotransferase of pig heart. Biochem J. 1963 Jul;88:172–175. doi: 10.1042/bj0880172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandurra R., Cannella C. Mitochondrial aspartate aminotransferase from beef kidney. Eur J Biochem. 1972 Mar 27;26(2):196–206. doi: 10.1111/j.1432-1033.1972.tb01757.x. [DOI] [PubMed] [Google Scholar]
- Shrawder E. J., Martinez-Carrion M. Distribution and turnover of the heart and liver isoenzymes of aspartate transaminase in the developing and young chick. J Biol Chem. 1973 Mar 25;248(6):2147–2152. [PubMed] [Google Scholar]
- TURANO C., FASELLA P., GIARTOSIO A., VECCHINI P. [On the mechanism of action of glutamic-aspartic transaminase: kinetic studies]. Boll Soc Ital Biol Sper. 1960 Dec 31;36:1968–1972. [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- WADA H., MORINO Y. COMPARATIVE STUDIES ON GLUTAMIC-OXALACETIC TRANSAMINASES FROM THE MITOCHONDRIAL AND SOLUBLE FRACTIONS OF MAMMALIAN TISSUES. Vitam Horm. 1964;22:411–444. doi: 10.1016/s0083-6729(08)60346-5. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]