Abstract
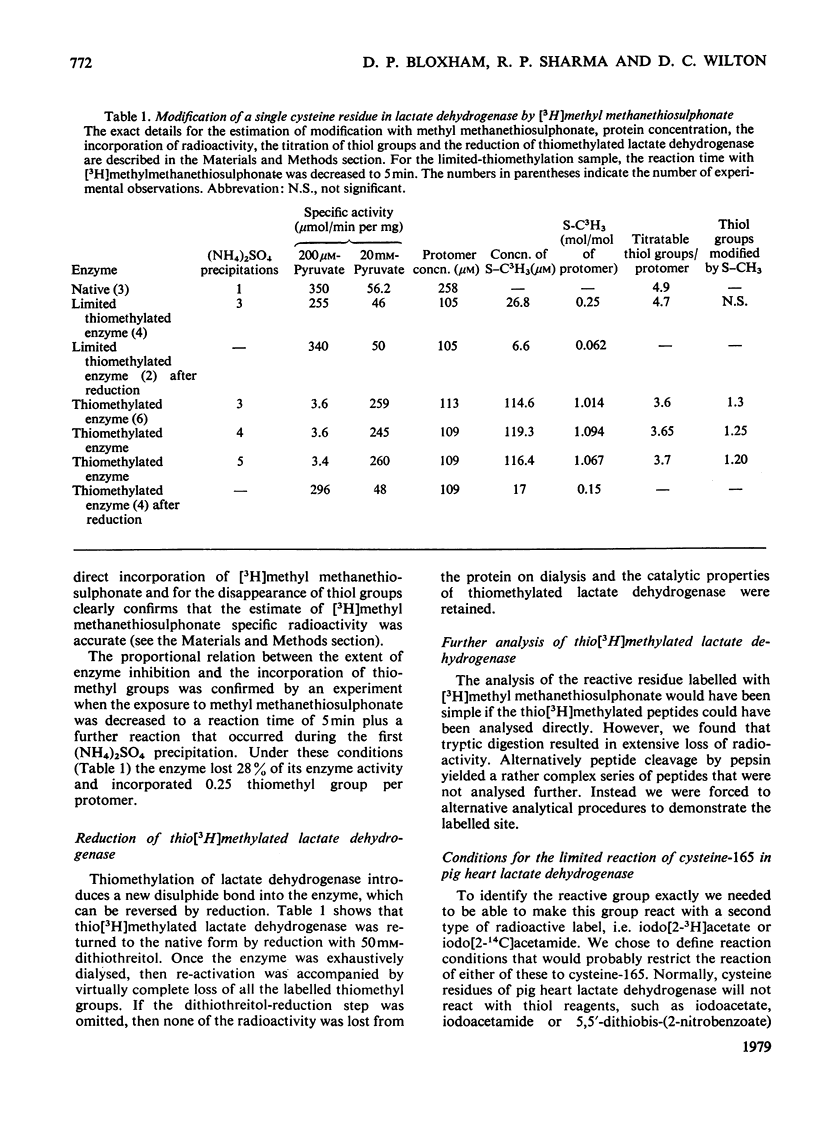
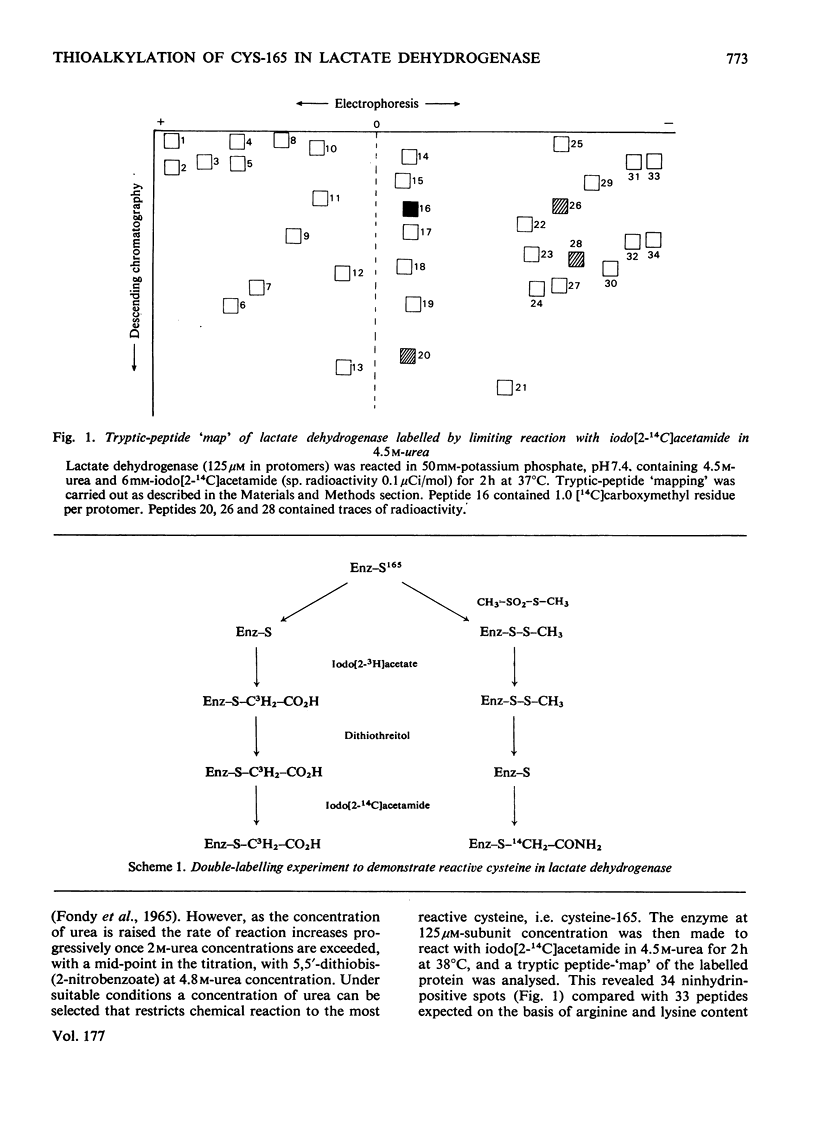
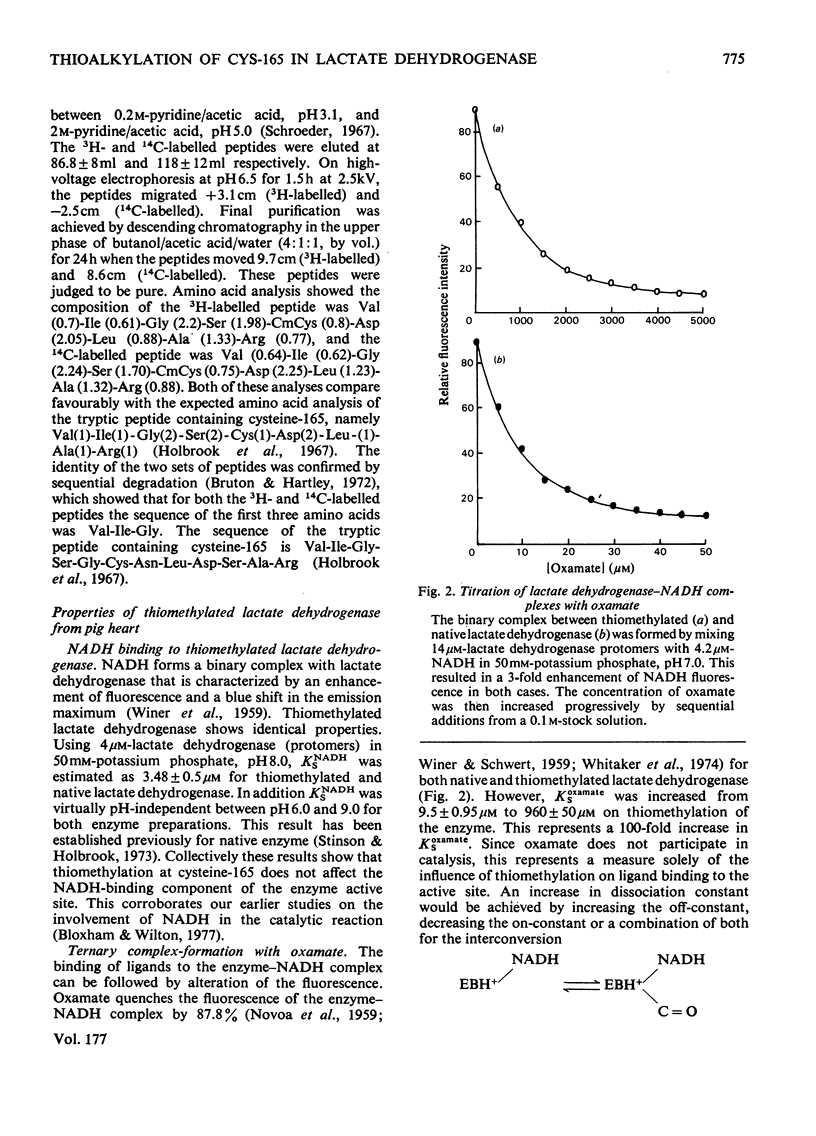
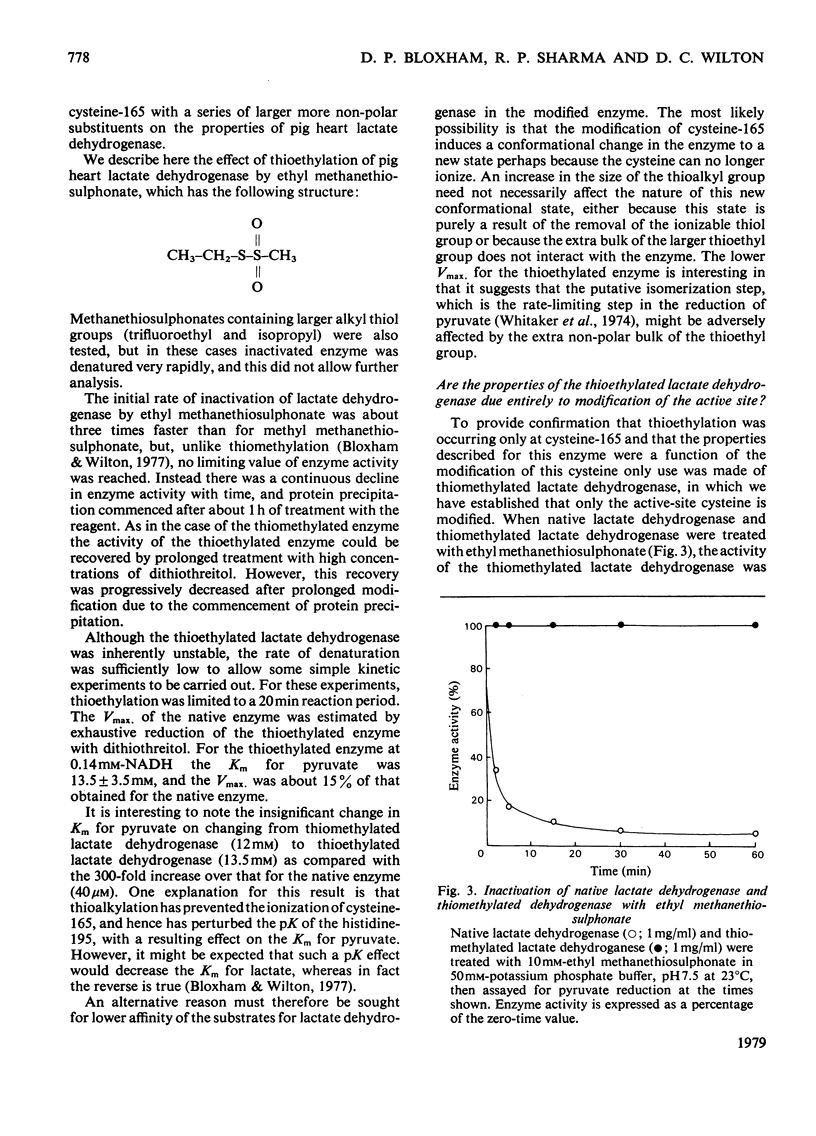
The reaction of pig heart lactate dehydrogenase with methyl methanethiosulphonate resulted in the modification of one thiol group per protomer, and this was located at cysteine-165 in the enzyme sequence. On reduction, both the thiomethylation of cysteine-165 and any changes in kinetic properties of the enzyme were completely reversed. Cysteine-165 has been considered essential for catalytic activity; however, cysteine-165-thiomethylated dehydrogenase possessed full catalytic activity, although the affinity of the enzyme for carbonyl-or hydroxy-containing substrates was markedly decreased. The nicotinamide nucleotide-binding capacity was unaffected, as judged by the formation of fluorescent complexes with NADH. The enzyme-mediated activation of NAD+, as judged by sulphite addition, was unaffected in thiomethylated lactate dehydrogenase. However, the affinity of oxamate for the enzyme--NADH complex was decreased by 100-fold and it was calculated that this constituted a net increase of 10.4 kJ/mol in the activation energy for binding. Thiomethylated lactate dehydrogenase was able to form an abortive adduct between NAD+ and fluoropyruvate. However, the equilibrium constant for adduct formation between pyruvate and NAD+ was too low to demonstrate this complex at reasonable pyruvate concentrations. A conformational change in the protein structure on selective thiomethylation was revealed by the decreased thermostability of the modified enzyme. The alteration of lactate dehydrogenase catalytic properties on modification depended on the bulk of the reagent used, since thioethylation resulted in an increase in Km for pyruvate (13.5 +/- 3.5 mm) and an 85% decrease in maximum catalytic activity. The implications of all these findings for the catalytic mechanism of lactate dehydrogenase are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Ford G. C., Koekoek R., Lentz P. J., McPherson A., Jr, Rossmann M. G., Smiley I. E., Schevitz R. W., Wonacott A. J. Structure of lactate dehydrogenase at 2-8 A resolution. Nature. 1970 Sep 12;227(5263):1098–1103. doi: 10.1038/2271098a0. [DOI] [PubMed] [Google Scholar]
- Arnold L. J., Jr, Kaplan N. O. The structure of the abortive diphosphopyridine nucleotide-pyruvate-lactate dehydrogenase ternary complex as determined by proton magnetic resonance analysis. J Biol Chem. 1974 Jan 25;249(2):652–655. [PubMed] [Google Scholar]
- Bloxham D. P., Coghlin S. J., Sharma R. P. Use of methanethiolation to investigate the catalytic role of sulphydryl groups in rabbit skeletal muscle pyruvate kinase. Biochim Biophys Acta. 1978 Jul 7;525(1):61–73. doi: 10.1016/0005-2744(78)90200-0. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Giles I. G., Wilton D. C., Akhtar M. The mechanism of the bond forming events in pyridine nucleotide linked oxidoreductases. Studies with epoxide inhibitors of lactic dehydrogenase and beta-hydroxybutyrate dehydrogenase. Biochemistry. 1975 May 20;14(10):2235–2241. doi: 10.1021/bi00681a030. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Wilton D. C. Modification of pig heart lactate dehydrogenase with methyl methanethiosulphonate to produce an enzyme with altered catalytic activity. Biochem J. 1977 Mar 1;161(3):643–651. doi: 10.1042/bj1610643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Copper A. J., Redfield A. G. Proton magnetic resonance studies of alpha-keto acids. J Biol Chem. 1975 Jan 25;250(2):527–532. [PubMed] [Google Scholar]
- Di Sabato G. Adducts of diphosphopyridine nucleotide and carbonyl compounds. Biochemistry. 1970 Nov 10;9(23):4594–4600. doi: 10.1021/bi00825a020. [DOI] [PubMed] [Google Scholar]
- EISMAN E. H., LEE H. A., Jr, WINER A. D. STUDIES ON FLUOROPYRUVATE AS A SUBSTRATE OF LACTATE DEHYDROGENASE. Biochemistry. 1965 Mar;4:606–610. doi: 10.1021/bi00879a034. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Everse J., Barnett R. E., Thorne C. J., Kaplan N. O. The formation of ternary complexes by diphosphopyridine nucleotide-dependent dehydrogenases. Arch Biochem Biophys. 1971 Apr;143(2):444–460. doi: 10.1016/0003-9861(71)90230-x. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- FROMM H. J. Evidence for ternary-complex formation with rabbit-muscle lactic acid dehydrogenase, diphosphopyridine nucleotide and pyruvic acid. Biochim Biophys Acta. 1961 Sep 2;52:199–200. doi: 10.1016/0006-3002(61)90919-2. [DOI] [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J. Direct measurement of proton binding to the active ternary complex of pig heart lactate dehydrogenase. Biochem J. 1973 Aug;133(4):847–849. doi: 10.1042/bj1330847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Gutfreund H. Approaches to the study of enzyme mechanisms lactate dehydrogenase. FEBS Lett. 1973 Apr 15;31(2):157–169. doi: 10.1016/0014-5793(73)80095-x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G. Bedeutung von SH-Gruppen für die enzymatische Aktivität. 3. Eine Methode, um die essentiellen Cysteinreste in nativer Schweineherz-Lactatdehydrogenase (Isozym I) radioaktiv zu markieren. Biochem Z. 1965 Jun 3;342(1):111–114. [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G., Mella K., Volz M., Leskowac W., Jeckel R. The importance of SH-groups for enzymic activity. 7. The amino acid sequence around the essential SH-group of pig heart lactate dehydrogenase, isoenzyme I. Eur J Biochem. 1967 Jun;1(4):476–481. doi: 10.1111/j.1432-1033.1967.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. The use of ternary complexes to study ionizations and isomerizations during catalysis by lactate dehydrogenase. Biochem J. 1973 Apr;131(4):739–748. doi: 10.1042/bj1310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapmeyer H., Pfleiderer G., Trommer W. E. A transition state analogue for two pyruvate metabolizing enzymes, lactate dehydrogenase and alanine dehydrogenase. Biochemistry. 1976 Nov 16;15(23):5024–5028. doi: 10.1021/bi00668a012. [DOI] [PubMed] [Google Scholar]
- Kapmeyer W., Pfleiderer G. Characterisation of a highly hydrophobically modified lactate dehydrogenase. Biochim Biophys Acta. 1977 Apr 12;481(2):328–339. doi: 10.1016/0005-2744(77)90266-2. [DOI] [PubMed] [Google Scholar]
- NEILANDS J. B. Studies on lactic dehydrogenase of heart. III. Action of inhibitors. J Biol Chem. 1954 May;208(1):225–230. [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Oxley S. S., Allison W. S., Kaplan N. O. Aminoacid sequence of dogfish M4 lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1790–1793. doi: 10.1073/pnas.70.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Oxley S. S. Homologies in the active site regions of lactate dehydrogenases. Arch Biochem Biophys. 1976 Aug;175(2):373–383. doi: 10.1016/0003-9861(76)90524-5. [DOI] [PubMed] [Google Scholar]
- WACHSMUTH E. D., PFLEIDERER G., WIELAND T. AMINOSAEUREZUSAMMENSETZUNG VON ISOZYMEN DER LACTATDEHYDROGENASE AUS MENSCHLICHEN UND TIERISCHEN ORGANEN. Biochem Z. 1964 Jul 8;340:80–94. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W., MILLAR D. B. Lactic dehydrogenase. VI. Fluorimetric measurements of the complex of enzyme and reduced diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1149–1154. [PubMed] [Google Scholar]
- Warren W. A. Catalysis of both oxidation and reduction of glyoxylate by pig heart lactate dehydrogenase isozyme 1. J Biol Chem. 1970 Apr 10;245(7):1675–1681. [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]


