Abstract
1. The aim of this work was to investigate why non-lactating dairy cows are less susceptible to the development of ketonaemia during food deprivation than are dairy cows in early lactation. 2. The first experiment (Expt. A) consisted of determining the effect of 6 days of food deprivation on the concentrations of ketone bodies, and of metabolites related to the regulation of ketogenesis, in jugular blood and liver of non-lactating cows. 3. During the food deprivation, blood ketone-body concentrations rose significantly, but to a value that was only 16% of that achieved in lactating cows deprived of food for 6 days [Baird, Heitzman & Hibbitt (1972) Biochem. J. 128, 1311--1318]. 4. In the liver, food deprivation caused: a rise in ketone-body concentrations; a fall in the concentration of glycogen and of various intermediates of the Embden-Meyerhof pathway and the tricarboxylic acid cycle; an increase in cytoplasmic reduction; a decrease in the [total NAD+]/[total NADH] ratio; a decrease in energy charge. These changes were all qualitatively similar to those previously observed in the livers of the food-deprived lactating cows. 5. There appeared therefore to be a discrepancy in the food-deprived non-lactating cows between the absence of marked ketonaemia and the occurrence of metabolic changes within the liver suggesting increased hepatic ketogenesis. This discrepancy was partially resolved in Expt. B by the observation in two catheterized non-lactating cows that, although there was a 2-fold increase in hepatic ketogenesis during 6 days of food deprivation, ketogenesis from the splanchnic bed as a whole (i.e. gut and liver combined) declined slightly owing to cessation of gut ketogenesis.
Full text
PDF


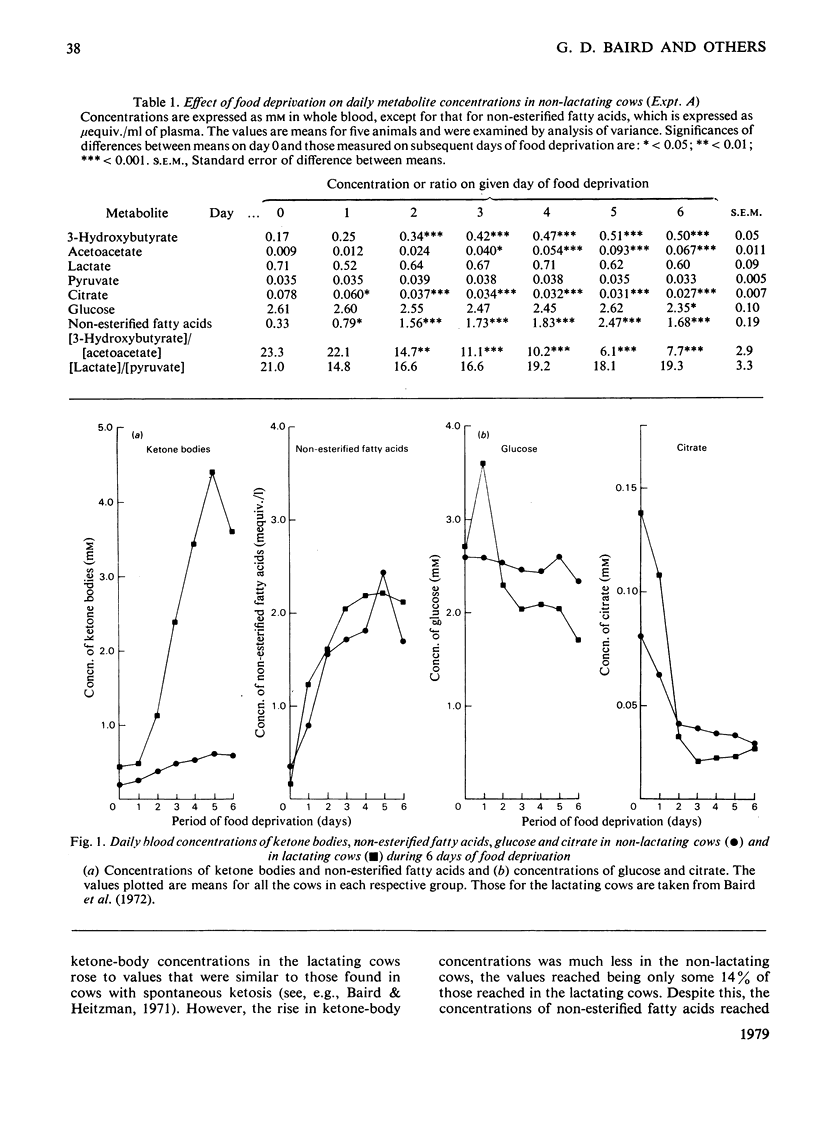
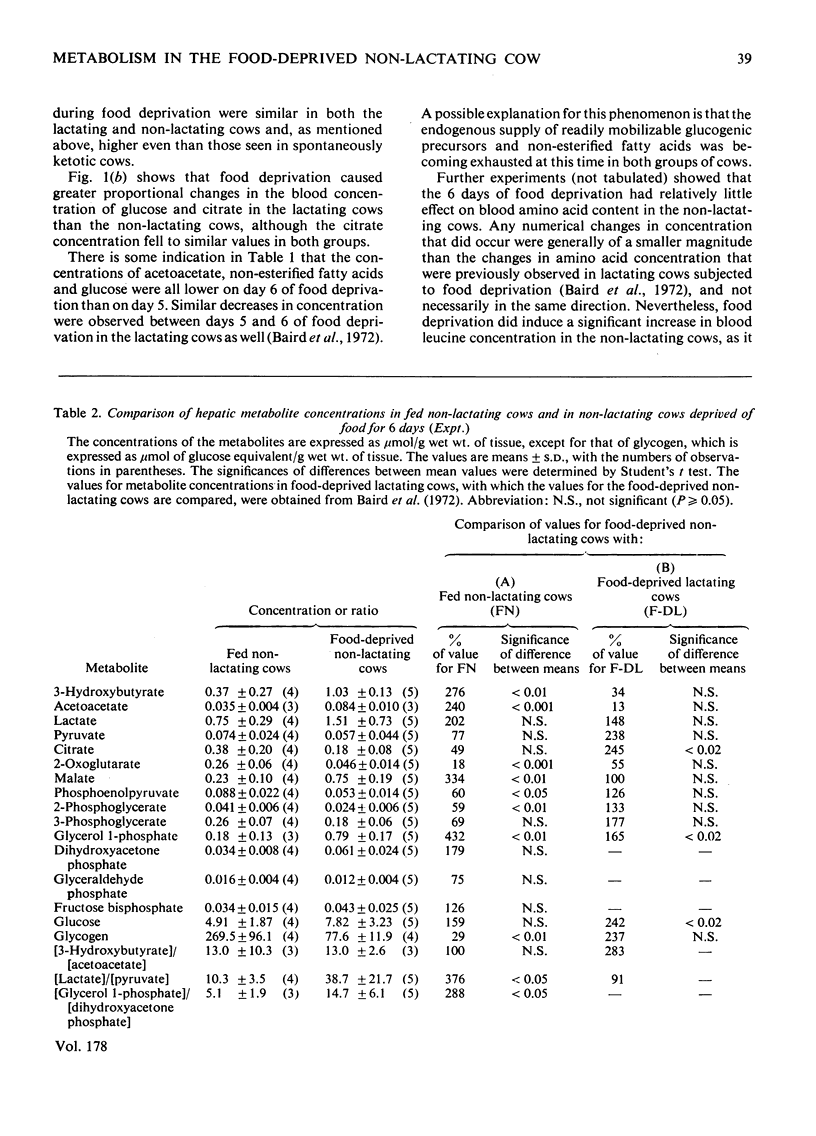

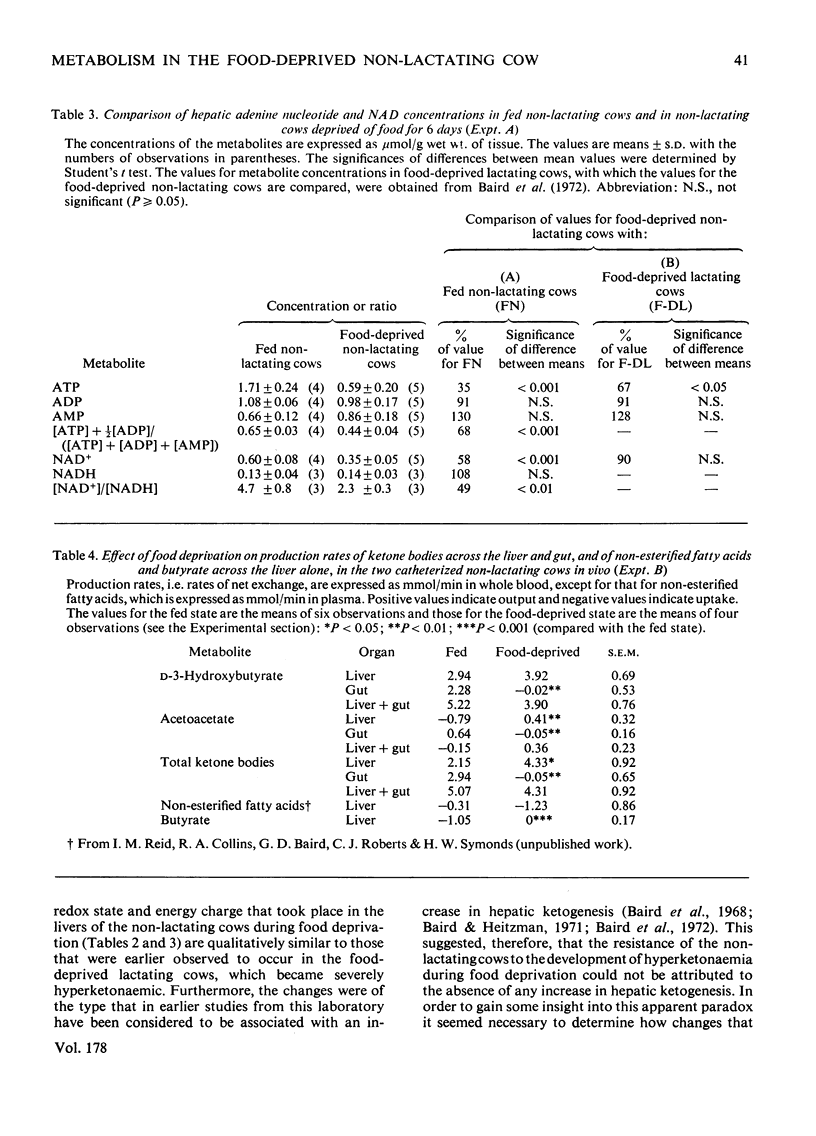



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Citrate and the citrate cycle in the regulation of energy metabolism. Biochem Soc Symp. 1968;27:23–40. [PubMed] [Google Scholar]
- Atkinson T., Fowler V. R., Garton G. A., Lough A. K. A rapid method for the accurate determination of lipid in animal tissues. Analyst. 1972 Jul;97(156):562–568. doi: 10.1039/an9729700562. [DOI] [PubMed] [Google Scholar]
- BERGMAN E. N., KON K. ACETOACETATE TURNOVER AND OXIDATION RATES IN OVINE PREGNANCY KETOSIS. Am J Physiol. 1964 Feb;206:449–452. doi: 10.1152/ajplegacy.1964.206.2.449. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Gluconeogenesis in the cow. The effects of a glucocorticoid on hepatic intermediary metabolism. Biochem J. 1970 Mar;116(5):865–874. doi: 10.1042/bj1160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J., Hibbitt K. G. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem J. 1972 Aug;128(5):1311–1318. doi: 10.1042/bj1281311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Mode of action of a glucocorticoid on bovine intermediary metabolism. Possible role in controlling hepatic ketogenesis. Biochim Biophys Acta. 1971 Oct;252(1):184–198. doi: 10.1016/0304-4165(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Hunter G. D. Biochemical aspects of bovine ketosis. Biochem J. 1968 May;107(5):683–689. doi: 10.1042/bj1070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberdorf F. A., Chernick S. S., Scow R. O. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest. 1970 Sep;49(9):1685–1693. doi: 10.1172/JCI106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Menzel P. H., Boden G., Owen O. E. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974 Oct;54(4):981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Jarrett I. G., Filsell O. H., Ballard F. J. Utilization of oxidizable substrates by the sheep hind limb: effects of starvation and exercise. Metabolism. 1976 May;25(5):523–531. doi: 10.1016/0026-0495(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Simultaneous measurements of hepatic and portal venous blood flow in the sheep and dog. Am J Physiol. 1969 Apr;216(4):946–952. doi: 10.1152/ajplegacy.1969.216.4.946. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Davidson L. M., Kim H. K. Quantitation of serum lipids by a simple TLC-charring method. Clin Chim Acta. 1973 Jun 14;46(1):63–68. doi: 10.1016/0009-8981(73)90103-4. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of ketogenesis and clinical aspects of the ketotic state. Metabolism. 1972 May;21(5):471–489. doi: 10.1016/0026-0495(72)90059-5. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Glucagon and ketogenesis. Metabolism. 1976 Nov;25(11 Suppl 1):1387–1389. doi: 10.1016/s0026-0495(76)80148-5. [DOI] [PubMed] [Google Scholar]
- Symonds H. W., Baird G. D. Cannulation of an hepatic vein, the portal vein and a mesenteric vein in the cow, and its use in the measurement of blood flow rates. Res Vet Sci. 1973 Mar;14(2):267–269. [PubMed] [Google Scholar]
- Treacher R. J., Baird G. D., Young J. L. Anti-ketogenic effect of glucose in the lactating cow deprived of food. Biochem J. 1976 Jul 15;158(1):127–134. doi: 10.1042/bj1580127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnam G. C., Jeacock M. K., Shepherd D. A. Activities of ketone body utilising enzymes in tissues of fed and fasted sheep. Res Vet Sci. 1978 Jan;24(1):124–125. [PubMed] [Google Scholar]
- Wieland O., Weiss L., Eger-Neufeldt I. Enzymatic regulation of liver acetyl-CoA metabolism in relation to ketogenesis. Adv Enzyme Regul. 1964;2:85–99. doi: 10.1016/s0065-2571(64)80007-8. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]


