Abstract
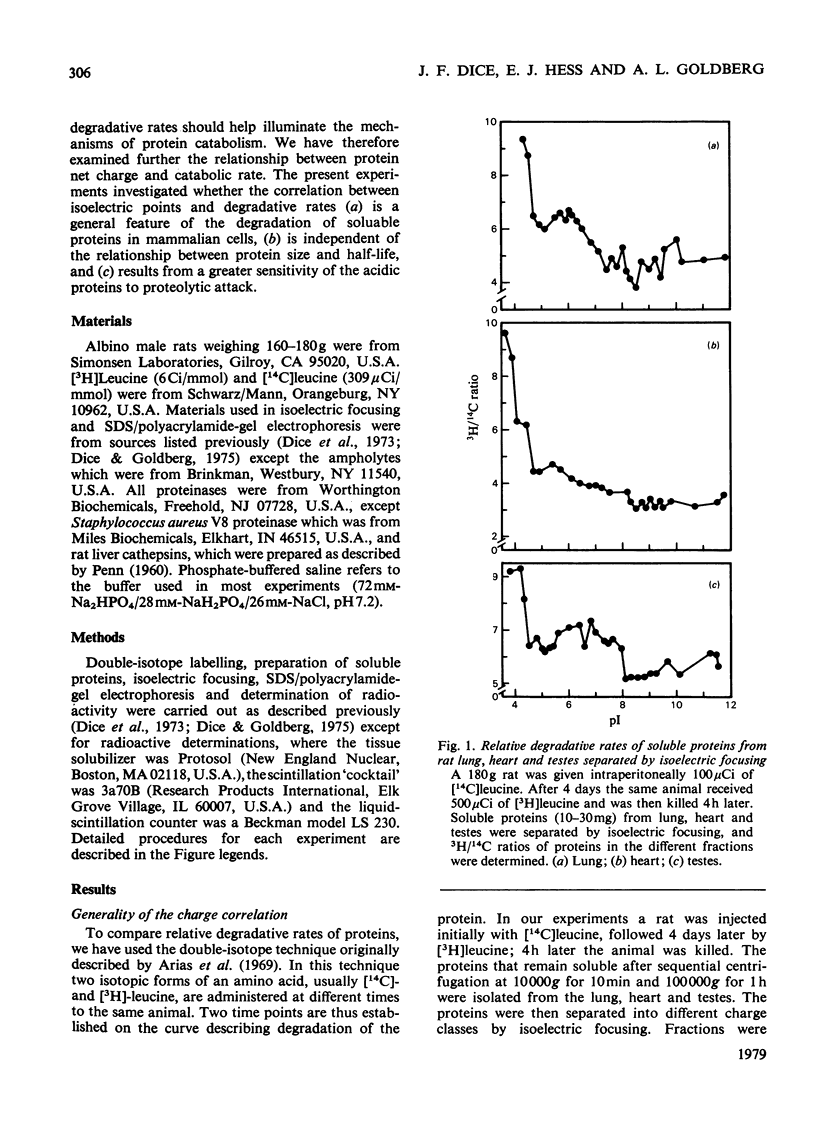
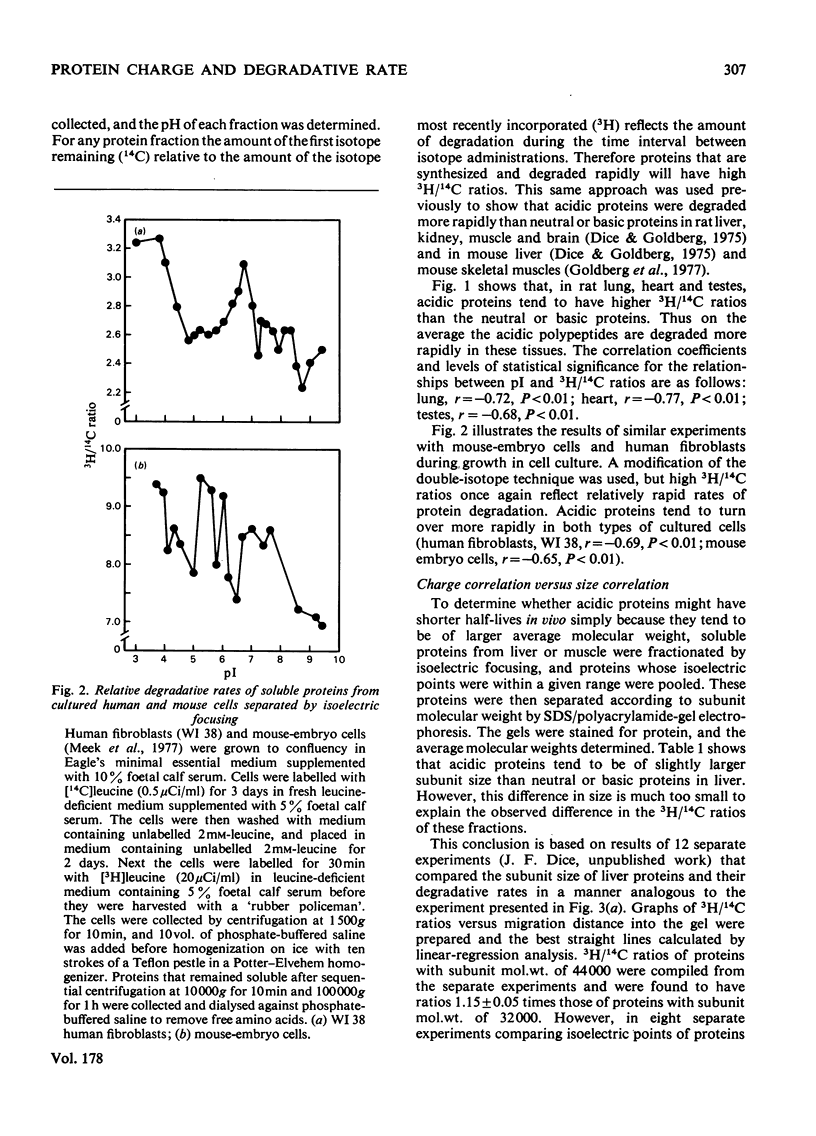
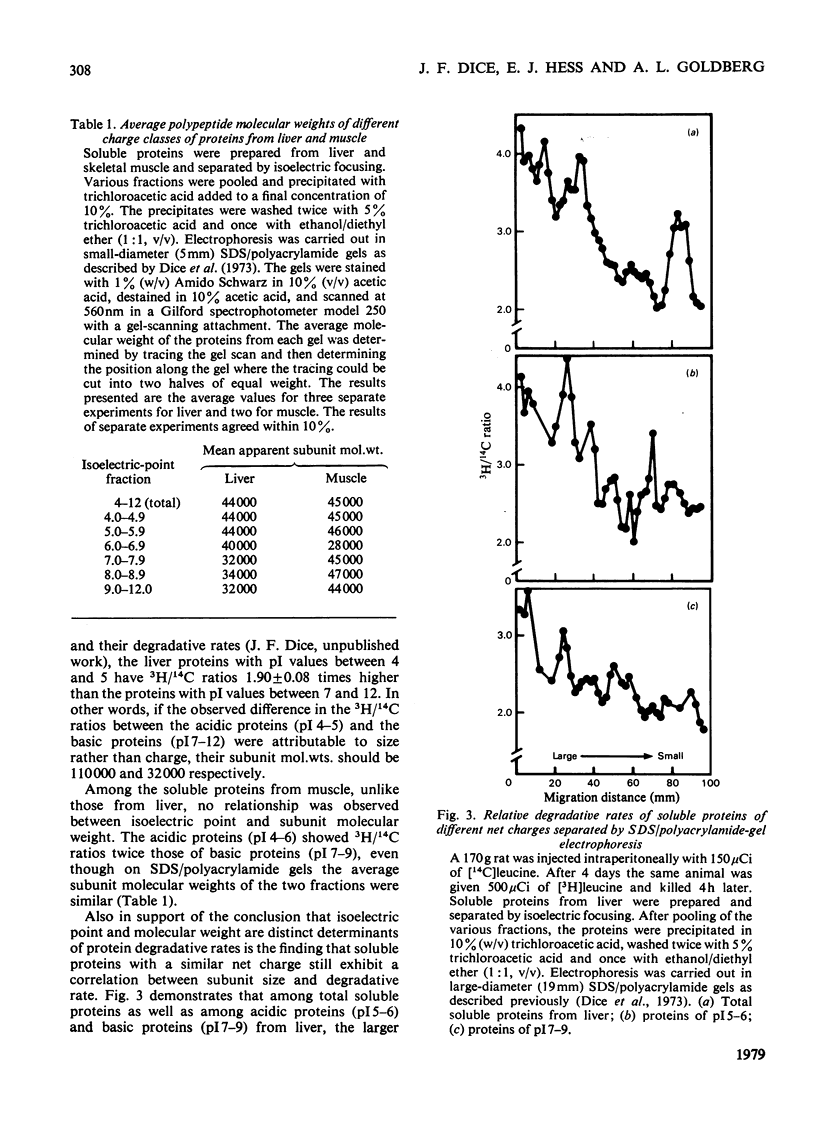
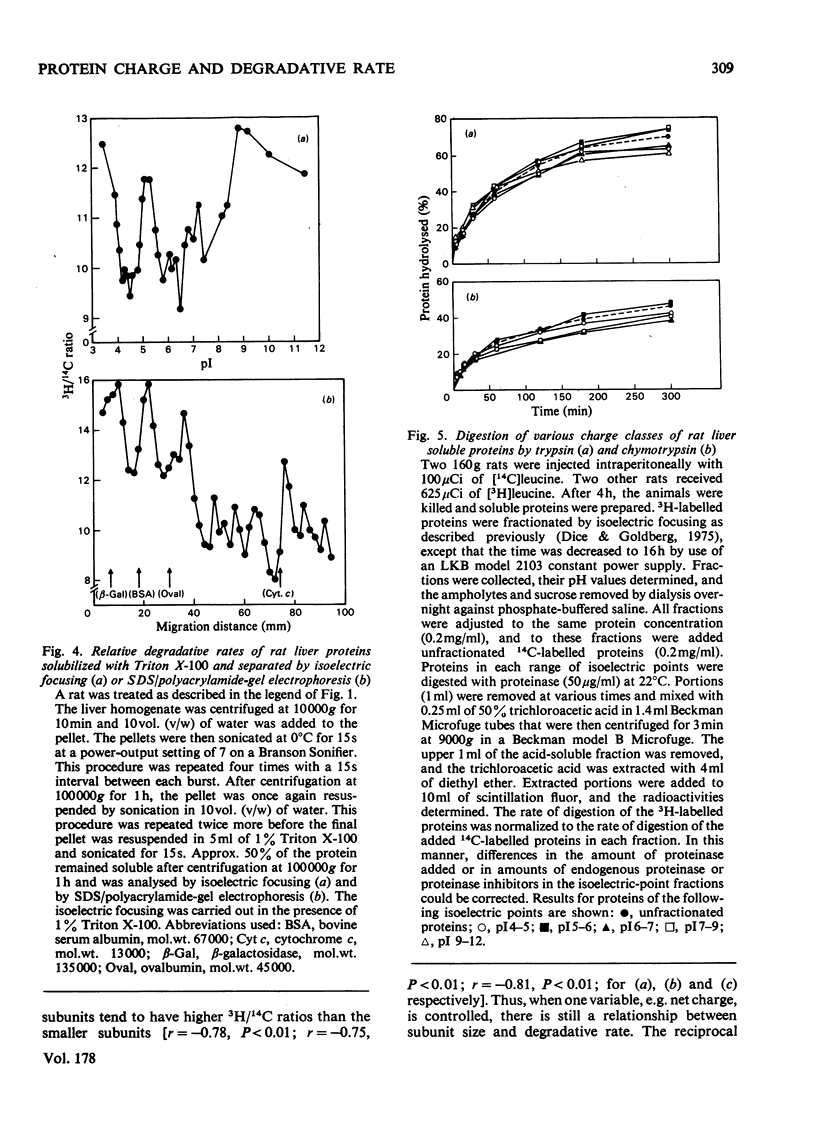
Acidic proteins tend to be degraded more rapidly than neutral or basic proteins in rat liver, skeletal muscle, kidney and brain and in mouse liver and skeletal muscle. We now report a similar relationship among soluble proteins from rat lung, heart and testes, and from human fibroblasts and mouse-embryo cells grown in culture. These findings indicate that the correlation between protein net charge and degradative rate is a general characteristic of intracellular protein degradation in mammals. This relationship between isoelectric point and half-life appears to be distinct from the previously reported correlation between subunit molecular weight and protein half-lives. The more rapid degradation of acidic proteins does not result from their being of larger molecular weight than neutral or basic proteins. Furthermore, proteins within specific isoelectric point ranges still exhibit a relationship between subunit size and half-life. Finally, a group of membrane or organelle-associated proteins that are insoluble in phosphate-buffered saline and water but soluble in 1% Triton X-100 exhibit a correlation between size and half-life, but not between net charge and half-life. The biochemical reasons for the relationship between protein isoelectric point and half-life are unclear, although several possible explanations are presented. It is not due to a greater sensitivity of acidic proteins to proteolytic attack since experiments with a variety of endoproteinases, including trypsin, chymotrypsin, Pronase, papain, chymopapain, Staphylococcus aureus V8 proteinase, pepsin and lysosomal cathepsins from rat liver, have failed to demonstrate more rapid digestion of acidic proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Concerning a possible mechanism for selective capture of cytoplasmic proteins by lysosomes. Biochem Biophys Res Commun. 1975 Nov 17;67(2):604–609. doi: 10.1016/0006-291x(75)90855-4. [DOI] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Effect of size on the relative rate of degradation of rat liver soluble proteins. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1473–1480. doi: 10.1016/0006-291x(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Fan W. W., Ivarie R. D., Levinson B. B. Nucleus-dependent regulation of tyrosine aminotransferase degradation in hepatoma tissue culture cells. J Biol Chem. 1977 Nov 10;252(21):7834–7841. [PubMed] [Google Scholar]
- Goldberg A. L. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2640–2644. doi: 10.1073/pnas.69.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Bowman P. D., Daniel C. W. Establishment of mouse embryo cells in vitro. Relationship of DNA synthesis, senescence and malignant transformation. Exp Cell Res. 1977 Jul;107(2):277–284. doi: 10.1016/0014-4827(77)90350-0. [DOI] [PubMed] [Google Scholar]
- Milman G., Lee E., Ghangas G. S., McLaughlin J. R., George M., Jr Analysis of HeLa cell hypoxanthine phosphoribosyltransferase mutants and revertants by two-dimensional polyacrylamide gel electrophoresis: evidence for silent gene activation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4589–4593. doi: 10.1073/pnas.73.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momany F. A., Aguanno J. J., Larrabee A. R. Correlation of degradative rates of proteins with a parameter calculated from amino acid composition and subunit size. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3093–3097. doi: 10.1073/pnas.73.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya J., Vigne J. L., De Castro F. T. The dynamic state of Tetrahymena pyriformis cytosol proteins during culture development. FEBS Lett. 1977 Apr 15;76(2):269–273. doi: 10.1016/0014-5793(77)80166-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- PENN N. W. The requirements for serum albumin metabolism in subcellular fractions of liver and brain. Biochim Biophys Acta. 1960 Jan 1;37:55–63. doi: 10.1016/0006-3002(60)90078-0. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. Studies of the stability in vivo and in vitro of rat liver tryptophan pyrrolase. J Biol Chem. 1965 Dec;240(12):4609–4620. [PubMed] [Google Scholar]
- Wilson D. L., Hall M. E., Stone G. C., Rubin R. W. Some improvements in two-dimensional gel electrophoresis of proteins. Protein mapping of eukaryotic tissue extracts. Anal Biochem. 1977 Nov;83(1):33–44. doi: 10.1016/0003-2697(77)90506-1. [DOI] [PubMed] [Google Scholar]


