Abstract
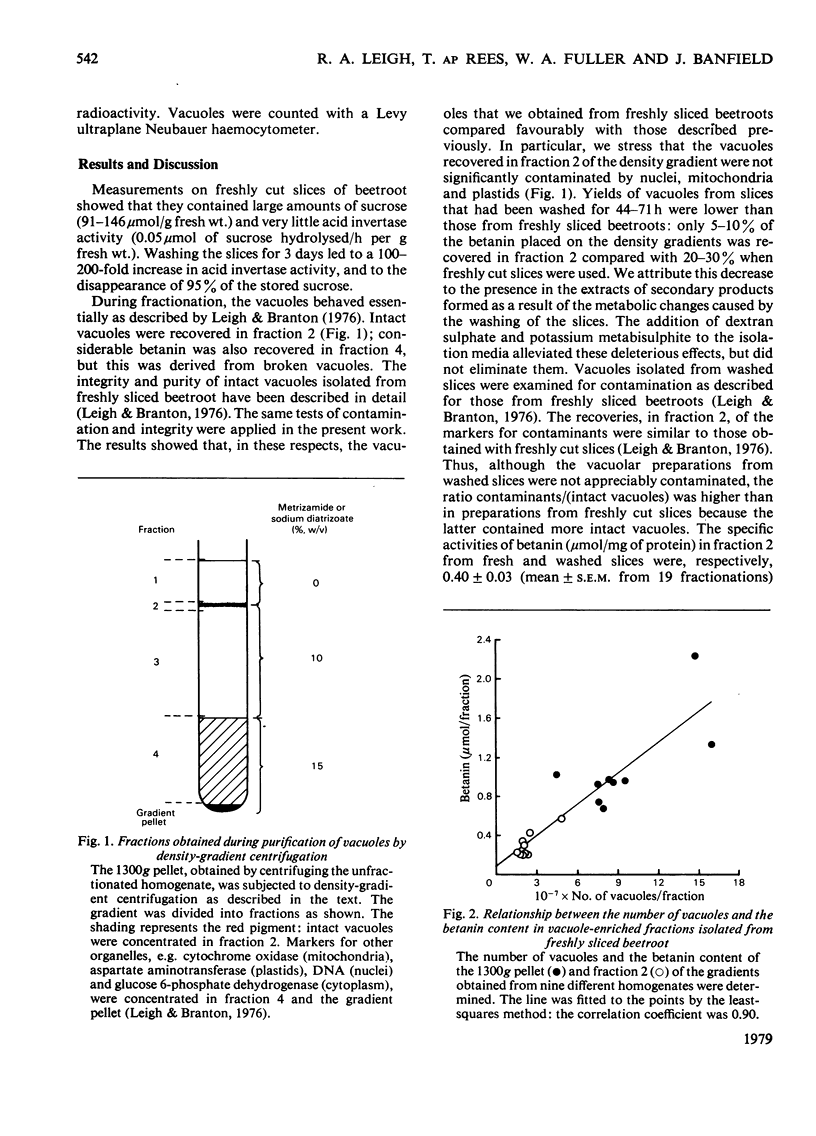
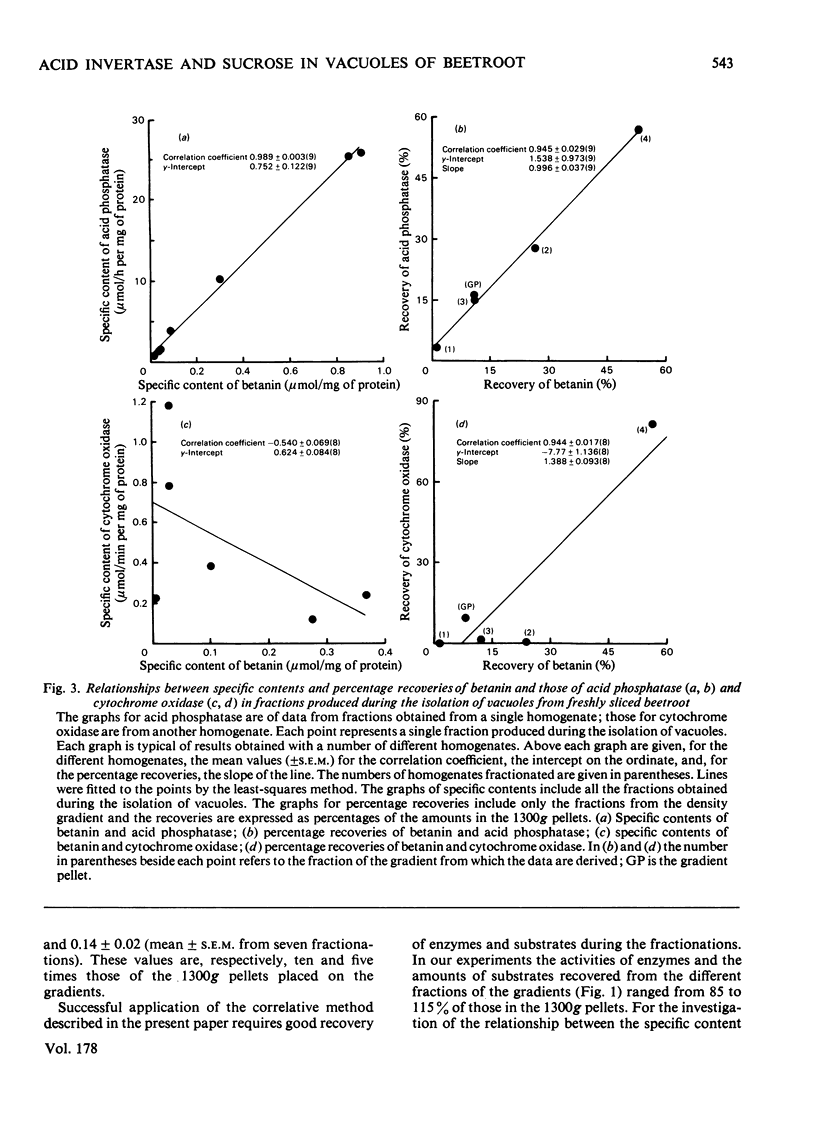
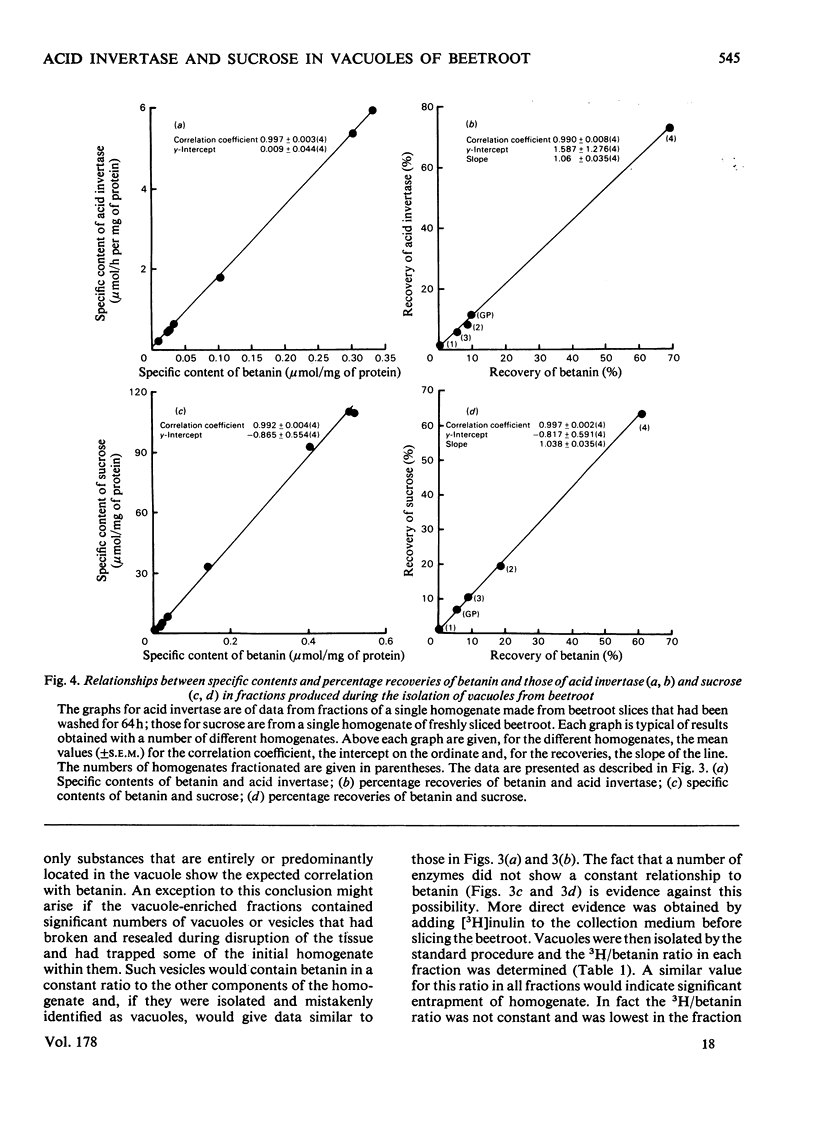
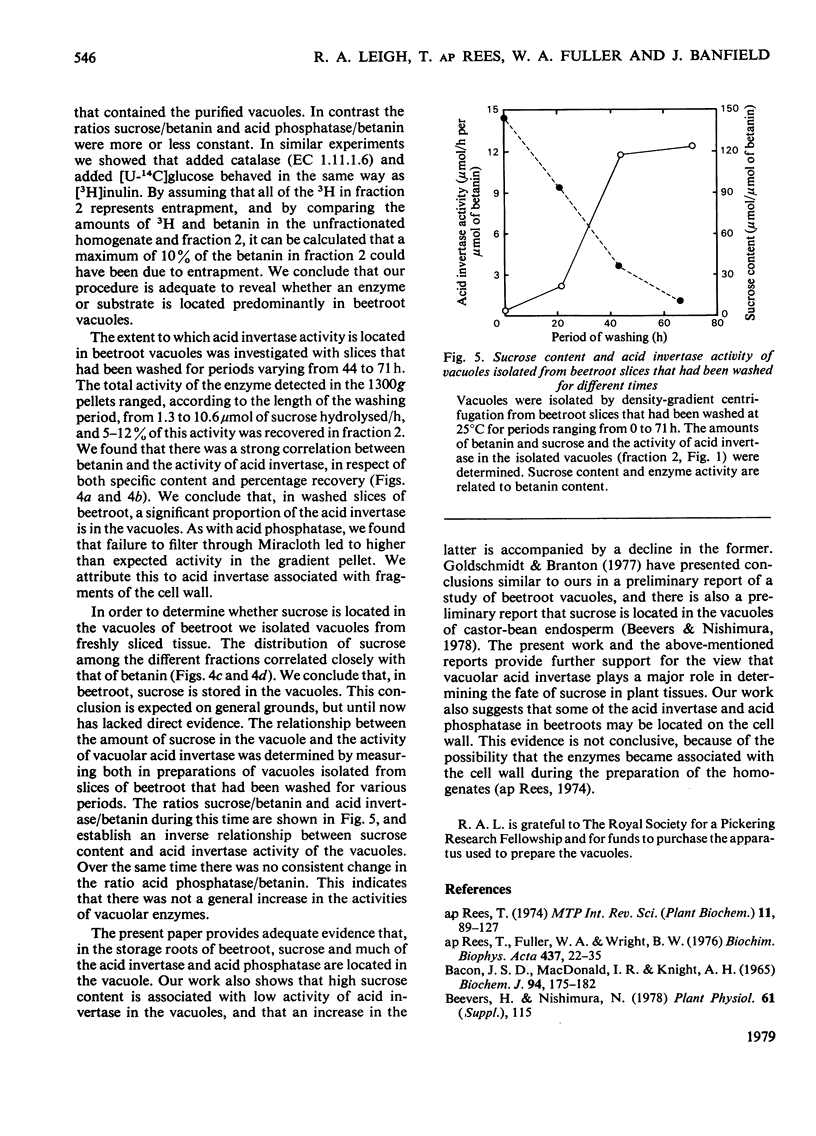
Vacuoles were isolated from freshly cut slices of the storage roots of beetroot (Beta vulgaris), and from slices that had been washed in aerated water for 1-3 days. The unique vacuolar location of betanin permitted the use of a correlative method to determine whether sucrose and acid invertase were located in the vacuoles. The specific content (the activity of the enzyme or amount of substrate per mg of protein) and the percentage recoveries for betanin, sucrose and acid invertase were determined for the different fractions obtained during the isolation of the vacuoles. For each fraction the specific content of betanin was plotted against those of sucrose and acid invertase. Similar correlative plots were drawn for the percentage recoveries. For both specific contents and percentage recoveries for correlation coefficients for sucrose and for acid invertase versus betanin were close to unity, and the lines passed near the origins. It is concluded that, in beetroot, most of the sucrose and much of the acid invertase are in the vacuoles. Measurements of vacuolar sucrose and acid invertase in beetroot slices washed for 1-3 days demonstrated an inverse relationship between sucrose content and acid invertase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON J. S., MACDONALD I. R., KNIGHT A. H. THE DEVELOPMENT OF INVERTASE ACTIVITY IN SLICES OF THE ROOT OF BETA VULGARIS L. WASHED UNDER ASEPTIC CONDITIONS. Biochem J. 1965 Jan;94:175–182. doi: 10.1042/bj0940175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C. Principles of tissue fractionation. J Theor Biol. 1964 Jan;6(1):33–59. doi: 10.1016/0022-5193(64)90065-7. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kilburn D. M., Taylor P. M. Effect of sulfhydryl reagents on glucose determination by the glucose oxidase method. Anal Biochem. 1969 Mar;27(3):555–558. doi: 10.1016/0003-2697(69)90069-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Meyer J., Matile P. H. Subcellular distribution of yeast invertase isoenzymes. Arch Microbiol. 1975 Mar 12;103(1):51–55. doi: 10.1007/BF00436329. [DOI] [PubMed] [Google Scholar]
- Rees T., Cerasi E., Wright B. W. Pathways of carbohydrate oxidation during thermogenesis by the spadix of Arum maculatum. Biochim Biophys Acta. 1976 Jun 23;437(1):22–35. doi: 10.1016/0304-4165(76)90344-5. [DOI] [PubMed] [Google Scholar]
- Sakano K., Komamine A. Change in the proportion of two aspartokinases in carrot root tissue in response to in vitro culture. Plant Physiol. 1978 Jan;61(1):115–118. doi: 10.1104/pp.61.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D., Macdonald I. R. Development of soluble and insoluble invertase activity in washed storage tissue slices. Plant Physiol. 1967 Mar;42(3):456–458. doi: 10.1104/pp.42.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson F. A., Morré D. J. Association of Phytochrome with Rough-surfaced Endoplasmic Reticulum Fractions from Soybean Hypocotyls. Plant Physiol. 1975 Dec;56(6):738–743. doi: 10.1104/pp.56.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]


