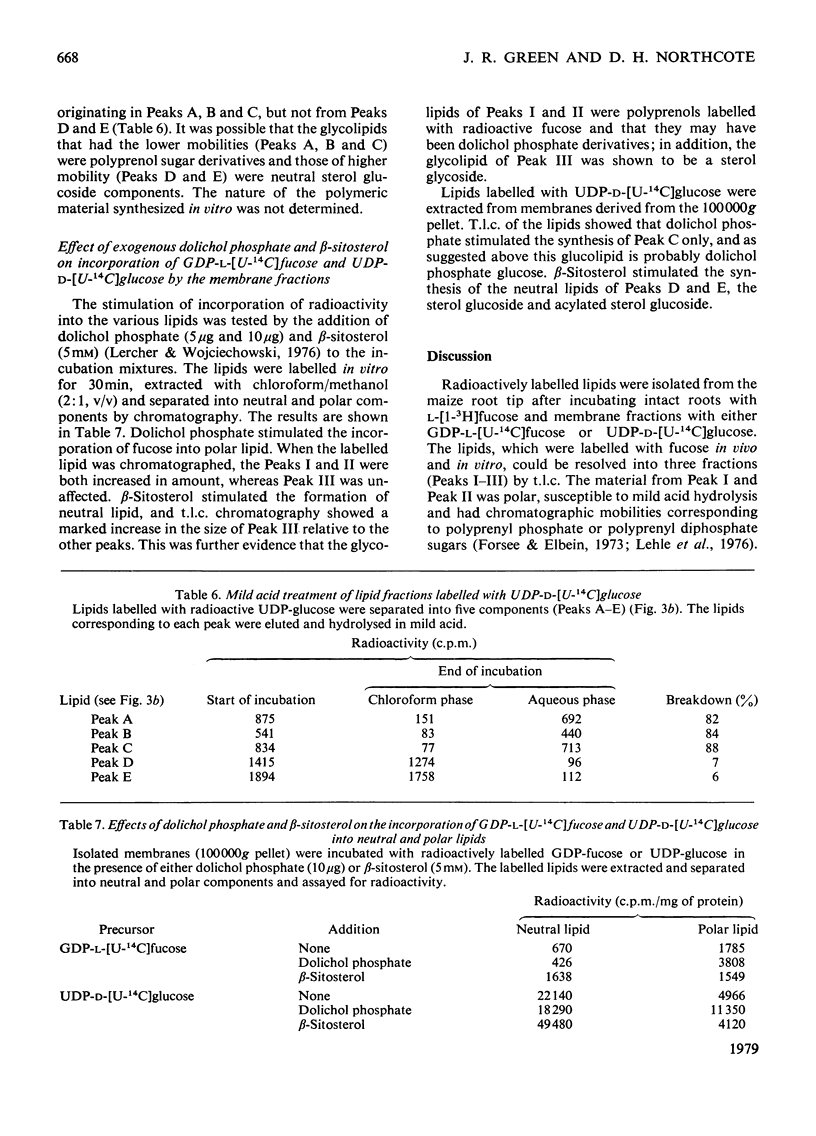
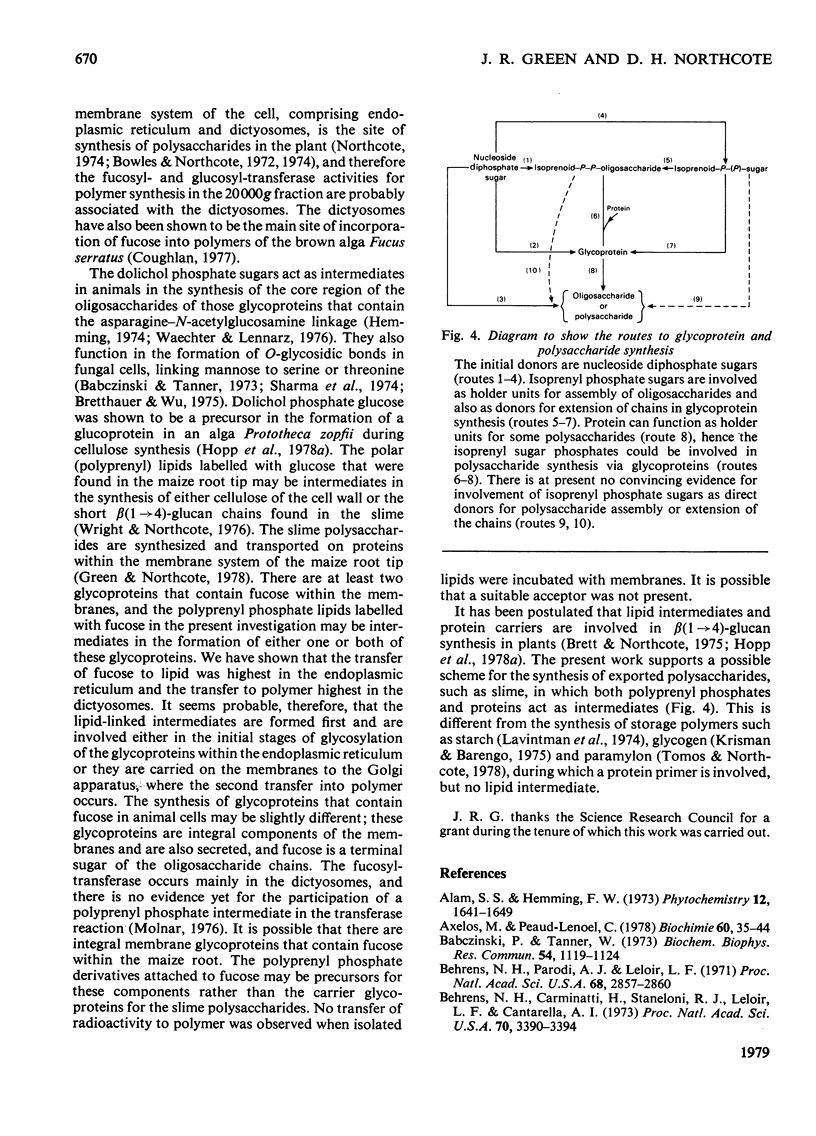
Abstract
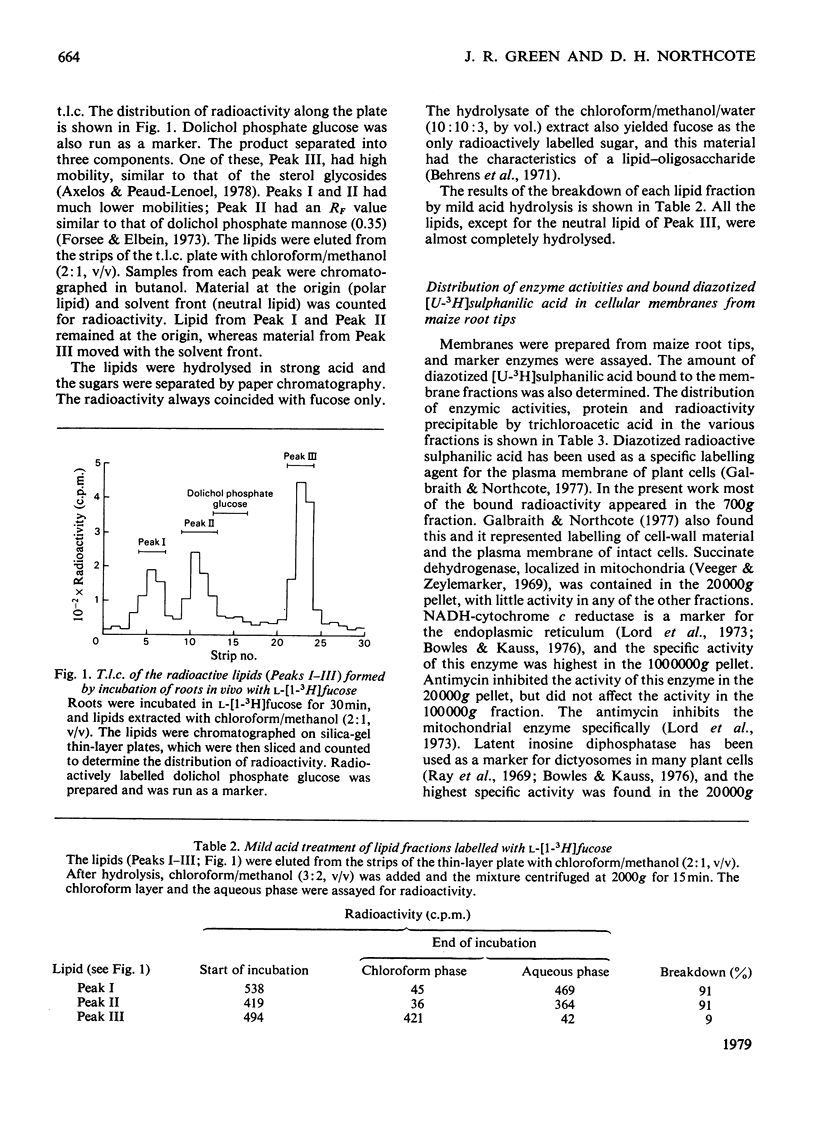
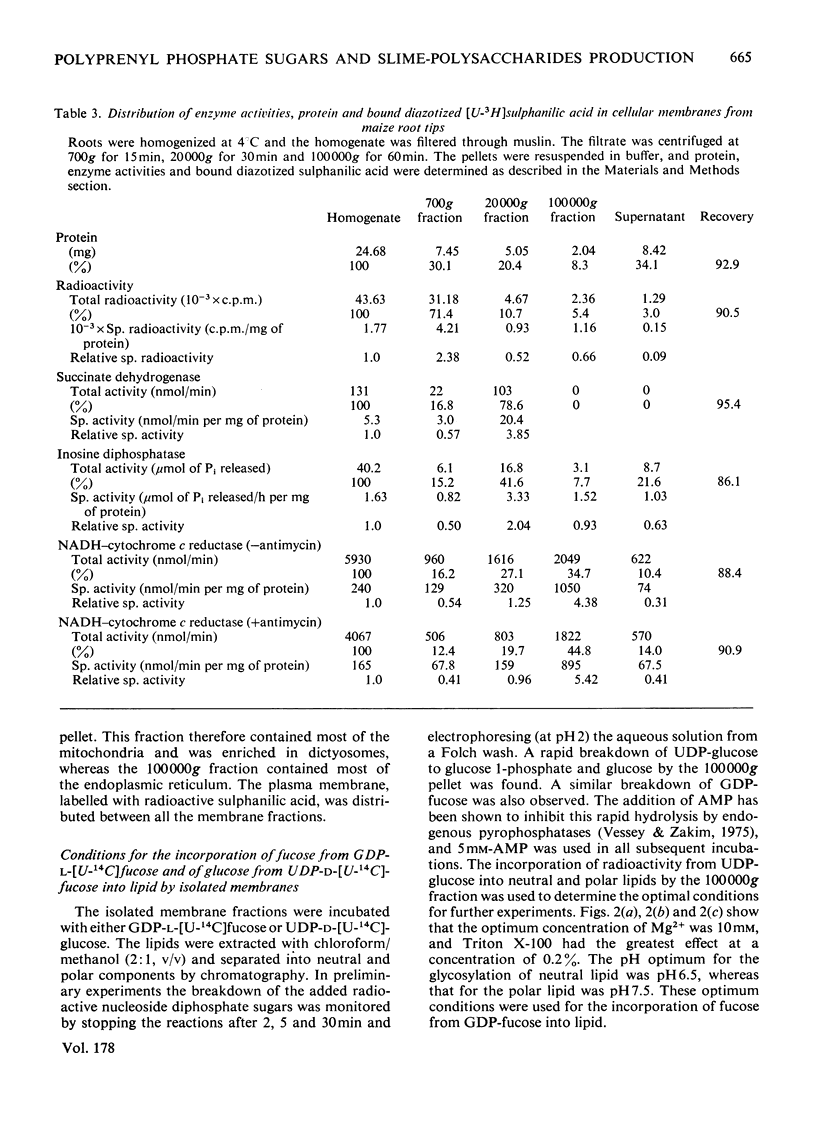
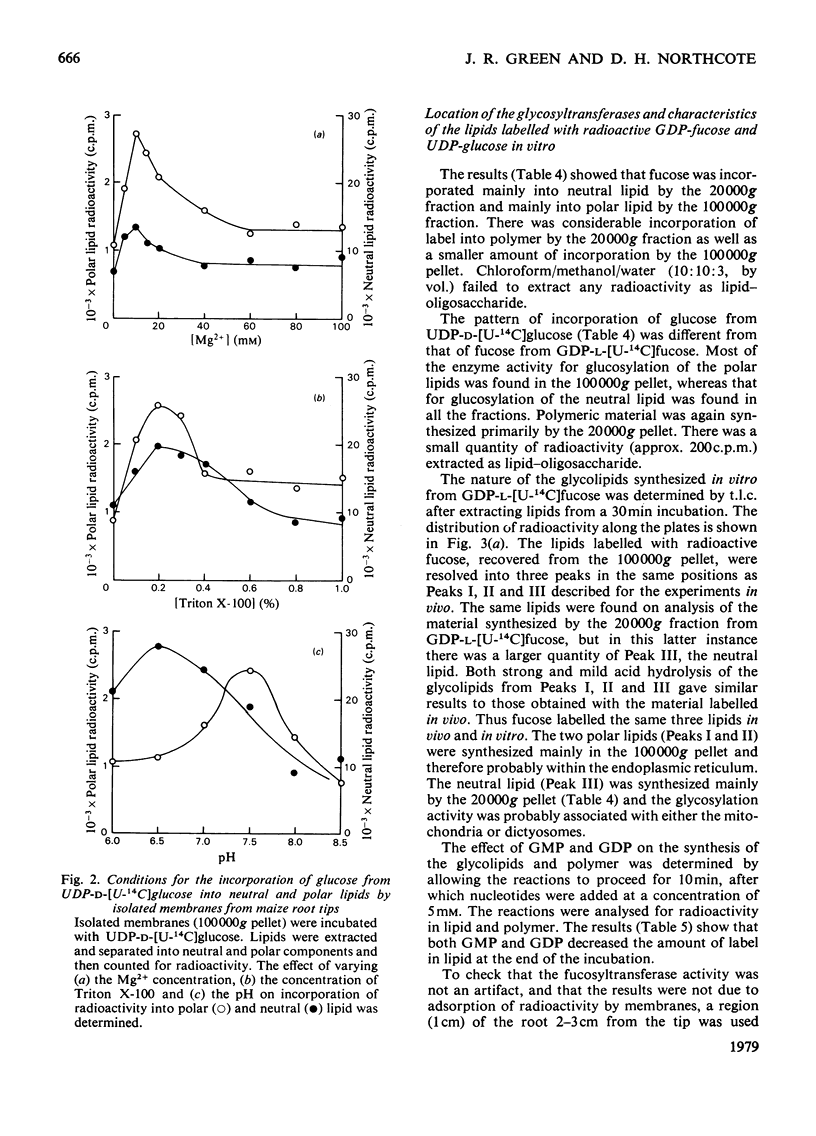
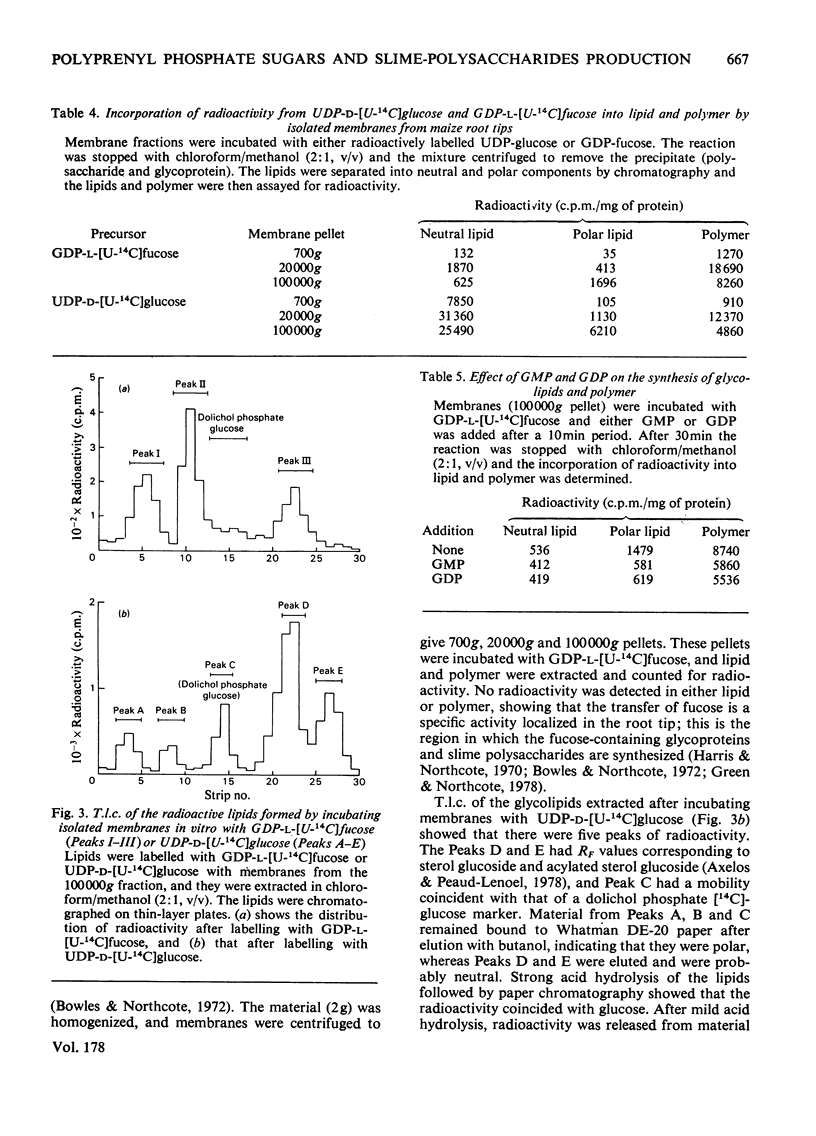
Two types of experiments were carried out; either maize roots were incubated in L-[1-3H]fucose or membranes were prepared from root tips and these were incubated with GDP-L-[U-14C]fucose or UDP-D-[U-4C]glucose. The radioactively labelled lipids that were synthesized in vivo and in vitro were extracted and separated into polar and neutral components. The polar lipids had the characteristics of polyprenyl phosphate and diphosphate fucose or glucose derivatives, and the neutral lipids of sterol glycosides (fucose or glucose). A partial separation of the glycolipid synthetase reactions was achieved. Membranes were fractionated into material that sedimented at 20,000g and 100,000g. Most of the polar glycolipid synthetase activity (for the incorporation of both fucose and glucose) was located in the 100,000 g pellet, and this activity was probably located in the endoplasmic reticulum. The neutral lipid, which contained fucose, was synthesized mainly by membranes of the 20,000g pellet, and the activity was probably associated with the dictyosomes, whereas the neutral glucolipids were synthesized by all the membrane fractions. It is suggested that the polar (polyprenyl) lipids labelled with fucose could act as possible intermediates during the synthesis of the glycoproteins and slime in the root tip.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelos M., Peaud-Lenoel C. Glycosyl transfers from UDP-sugars to lipids of plant membranes: identification and specificity of the transferases. Biochimie. 1978;60(1):35–44. doi: 10.1016/s0300-9084(78)80196-5. [DOI] [PubMed] [Google Scholar]
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Hirsh D. Synthesis of diazotized 35S sulfanilic acid of high specific activity: a label for the outer surface of cell membranes. Anal Biochem. 1975 Jun;66(2):629–631. doi: 10.1016/0003-2697(75)90630-2. [DOI] [PubMed] [Google Scholar]
- Bergman A., Mankowski T., Chojnacki T., De Luca L. M., Peterson E., Dallner G. Glycosyl transfer from nucleotide sugars to C85- and C55-polyprenyl and retinyl phosphates by microsomal subfractions and Golgi membranes of rat liver. Biochem J. 1978 Apr 15;172(1):123–127. doi: 10.1042/bj1720123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The amounts and rates of export of polysaccharides found within the membrane system of maize root cells. Biochem J. 1974 Jul;142(1):139–144. doi: 10.1042/bj1420139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C. T., Leloir L. F. Dolichyl monophosphate and its sugar derivatives in plants. Biochem J. 1977 Jan 1;161(1):93–101. doi: 10.1042/bj1610093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C. T., Northcote D. H. The formation of oligoglucans linked to lipid during synthesis of beta-glucan by characterized membrane fractions isolated from peas. Biochem J. 1975 Apr;148(1):107–117. doi: 10.1042/bj1480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretthauer R. K., Wu S. Synthesis of the mannosyl-O-serine (threonine) linkage of glycoproteins from polyisoprenylphosphate mannose in yeast (Hansenula holstii). Arch Biochem Biophys. 1975 Mar;167(1):151–160. doi: 10.1016/0003-9861(75)90451-8. [DOI] [PubMed] [Google Scholar]
- Coughlan S. Isolation and characterisation of a fucosyl transferase associated with dictysomes from the brown alga Fucus serratus L. FEBS Lett. 1977 Sep 1;81(1):33–36. doi: 10.1016/0014-5793(77)80921-6. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Glycoprotein biosynthesis in plants. Demonstration of lipid-linked oligosaccharides of mannose and N-acetylglucosamine. J Biol Chem. 1975 Dec 25;250(24):9283–9293. [PubMed] [Google Scholar]
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Green J. R., Northcote D. H. The structure and function of glycoproteins synthesized during slime-polysaccharide production by membranes of the root-cap cells of maize (Zea mays). Biochem J. 1978 Mar 15;170(3):599–608. doi: 10.1042/bj1700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp H. E., Romero P. A., Daleo G. R., Pont Lezica R. Synthesis of cellulose precursors. The involvement of lipid-linked sugars. Eur J Biochem. 1978 Mar 15;84(2):561–571. doi: 10.1111/j.1432-1033.1978.tb12199.x. [DOI] [PubMed] [Google Scholar]
- Hsu A. F., Baynes J. W., Heath E. C. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisman C. R., Barengo R. A precursor of glycogen biosynthesis: alpha-1,4-glucan-protein. Eur J Biochem. 1975 Mar 3;52(1):117–123. doi: 10.1111/j.1432-1033.1975.tb03979.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavintman N., Tandecarz J., Carceller M., Mendiara S., Cardini C. E. Role of uridine diphosphate glucose in the biosynthesis of starch. Mechanism of formation and enlargement of a glucoproteic acceptor. Eur J Biochem. 1974 Dec 16;50(1):145–155. doi: 10.1111/j.1432-1033.1974.tb03882.x. [DOI] [PubMed] [Google Scholar]
- Lehle L., Fartaczek F., Tanner W., Kauss H. Formation of polyprenol-linked mono- and oligosaccharides in Phaseolus aureus. Arch Biochem Biophys. 1976 Aug;175(2):419–426. doi: 10.1016/0003-9861(76)90529-4. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. Formation of lipid-bound oligosaccharides in yeast. Biochim Biophys Acta. 1975 Aug 13;399(2):364–374. doi: 10.1016/0304-4165(75)90265-2. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. Glycosyl transfer from dolichyl phosphate sugars to endogenous and exogenous glycoprotein acceptors in yeast. Eur J Biochem. 1978 Feb;83(2):563–570. doi: 10.1111/j.1432-1033.1978.tb12124.x. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Lezica R. P., Brett C. T., Martinez P. R., Dankert M. A. A glucose acceptor in plants with the properties of an alpha-saturated polyprenyl-monophosphate. Biochem Biophys Res Commun. 1975 Oct 6;66(3):980–987. doi: 10.1016/0006-291x(75)90736-6. [DOI] [PubMed] [Google Scholar]
- Lezica R. P. Membrane-bound UDP-Glucose: Lipid Glucosyltransferases from Peas. Plant Physiol. 1976 Nov;58(5):675–680. doi: 10.1104/pp.58.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Staneloni R., Cantarella A. I., Leloir L. F., Behrens N. H., Carminatti H., Levy J. A. Further studies on a glycolipid formed from dolichyl-D-glucosyl monophosphate. Carbohydr Res. 1973 Feb;26(2):393–400. doi: 10.1016/s0008-6215(00)84527-9. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C. B., Babczinski P., Lehle L., Tanner W. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):35–41. doi: 10.1111/j.1432-1033.1974.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B. K., White D. A. The formation of lipid-linked sugars as intermediates in glycoprotein synthesis in rabbit mammary gland. Biochem J. 1978 Feb 15;170(2):273–283. doi: 10.1042/bj1700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. III. Comparison of oligosaccharide-lipids formed by slices from several tissues. J Biol Chem. 1976 Oct 25;251(20):6420–6425. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. II. Studies on the structure of an oligosaccharide-lipid from thyroid. J Biol Chem. 1976 Oct 25;251(20):6409–6419. [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Utilization of exogenous GDP-mannose for the synthesis of mannose-containing lipids and glycoproteins by oviduct cells. J Biol Chem. 1976 Apr 25;251(8):2511–2519. [PubMed] [Google Scholar]
- Tomos A. D., Northcote D. H. A protein-glucan intermediate during paramylon synthesis. Biochem J. 1978 Jul 15;174(1):283–290. doi: 10.1042/bj1740283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey D. A., Lysenko N., Zakim D. Evidence for multiple enzymes in the dolichol utilizing pathway of glycoprotein biosynthesis. Biochim Biophys Acta. 1976 Mar 25;428(1):138–145. doi: 10.1016/0304-4165(76)90115-x. [DOI] [PubMed] [Google Scholar]
- Vessey D. A., Zakim D. Characterization of the reaction of GDP-mannose with dolichol phosphate in liver membranes. Eur J Biochem. 1975 May 6;53(2):499–504. doi: 10.1111/j.1432-1033.1975.tb04092.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lucas J. J., Lennarz W. J. Evidence for xylosyl lipids as intermediates in xylosyl transfers in hen oviduct membranes. Biochem Biophys Res Commun. 1974 Jan 23;56(2):343–350. doi: 10.1016/0006-291x(74)90848-1. [DOI] [PubMed] [Google Scholar]
- Wojciechowski Z. A., van Uon N. Intracellular localization and some properties of UDPG: sterol glucosyltransferase from Calendula officinalis. Acta Biochim Pol. 1975;22(1):25–38. [PubMed] [Google Scholar]
- Zatta P., Zakim D., Vessey D. A. The transfer of galactose from UDP-galactose to endogenous lipid acceptors in liver microsomes. Biochim Biophys Acta. 1975 Jun 12;392(2):361–365. doi: 10.1016/0304-4165(75)90018-5. [DOI] [PubMed] [Google Scholar]


