Abstract
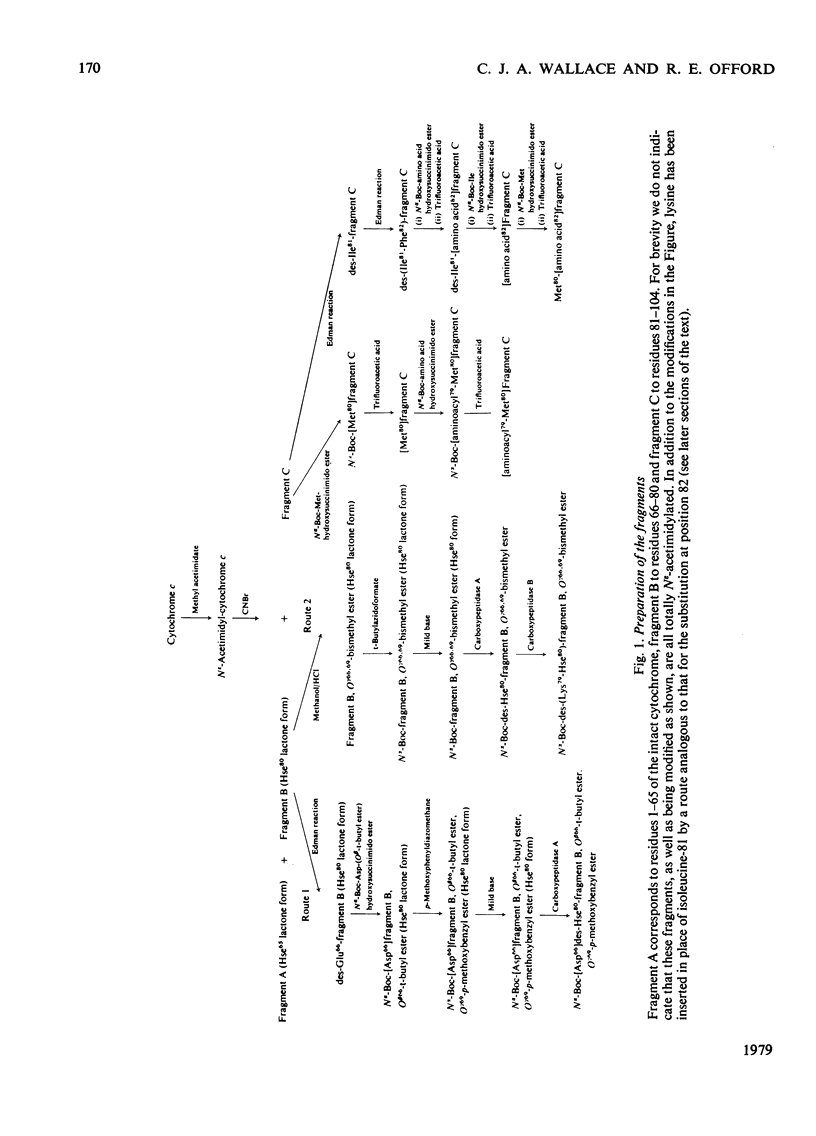
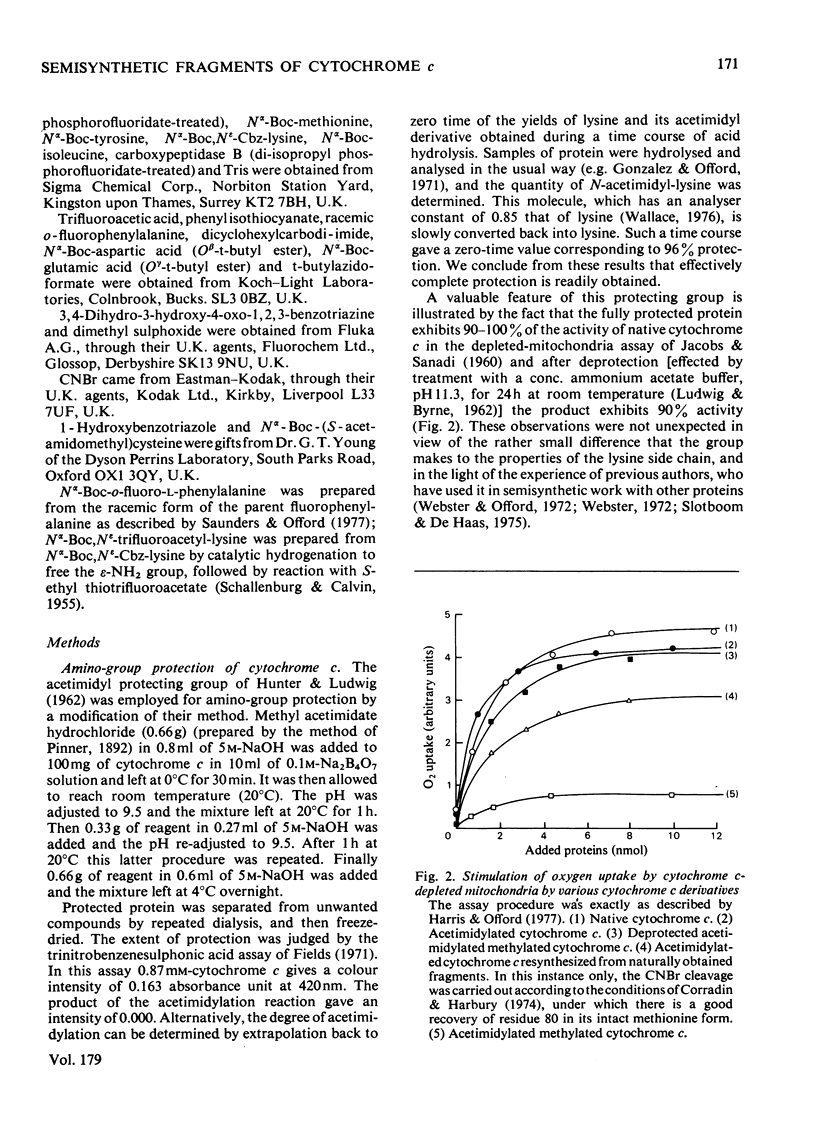
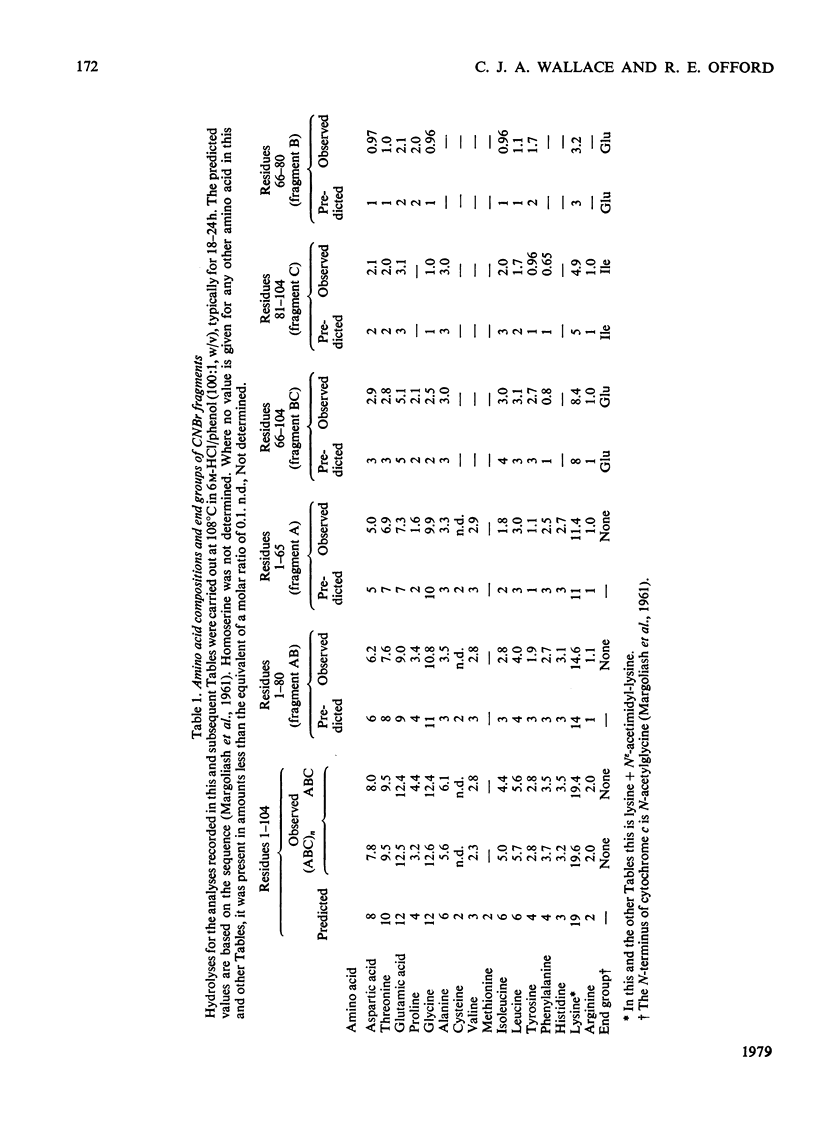
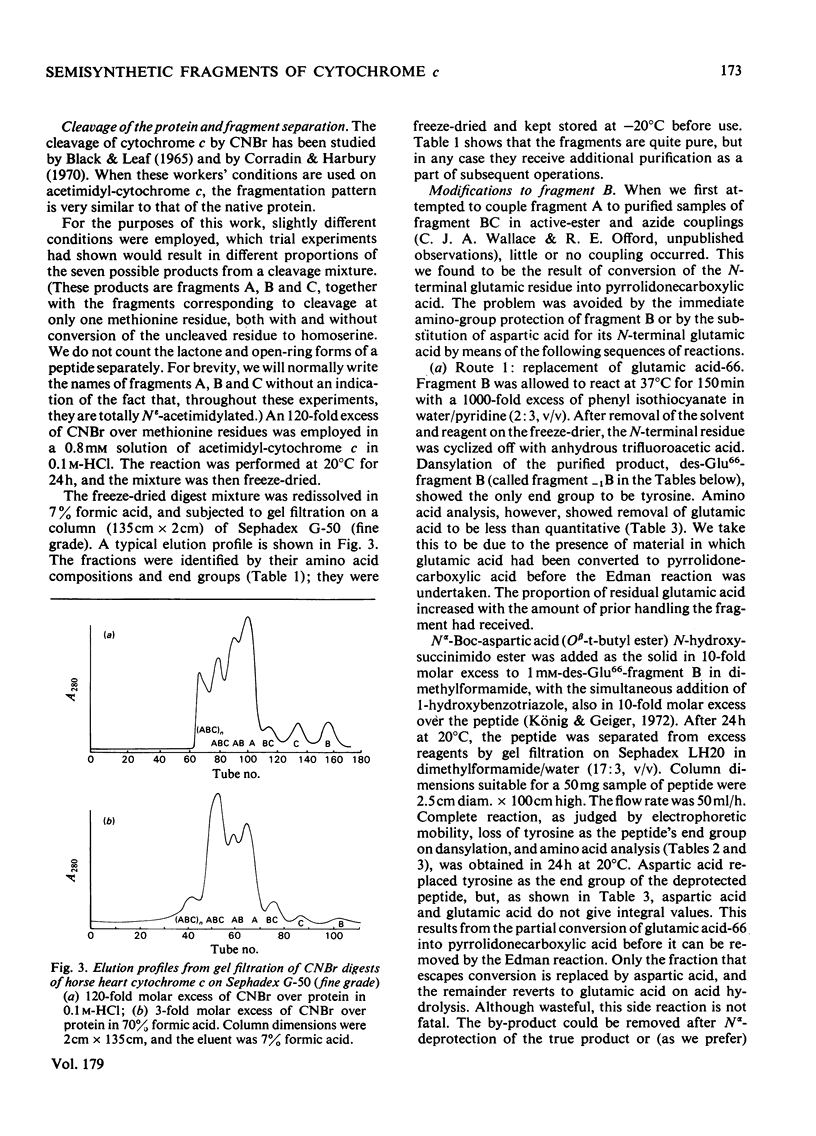
We describe the N epsilon-acetimidylation of horse heart cytochrome c with retention of biological activity, the cleavage of the modified protein by CNBr, the separation of the fragments, and their further side-chain protection. We describe the manipulation of the amino acid sequences of the fragments by stepwise semisynthetic methods. We have prepared fragments corresponding to residues 66-78 and 66-79 of the protein, as well as the [Asp66] analogue of fragment 66-79. We have prepared the natural sequence and the [o-fluoro-Phe82] analogue of the fragment corresponding to residues 81-104 of the protein, and the [N epsilon-trifluoroacetyl-Lys79], the [N epsilon-dinitrophenyl-Lys79] and the [S-acetamidomethyl-Cys79] analogues of fragment 79-104, and the [N epsilon-Cbz-Lys81] analogue of fragment 80-104. We have coupled back the fragments of natural sequence to form a semisynthetic fragment corresponding to residues 66-104 of the protein. Modified fragments were also coupled to give analogues of the 66-104-residue sequence. In every case the homoserine residue representing methionine-80 was removed from the C-terminus of the 66-80-residue fragment and replaced by methionine on the N-terminus of the 81-104 residue fragment during the preparation of the fragments for coupling. The semisynthetic fragments are ready for specific deprotection and further coupling. We have coupled one such fragment to the (1-65)-peptide to produce semisynthetic [Hse65]cytochrome c. The product has satisfactory characteristics on chemical analysis, and on assay of its biological activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstow L. E., Young R. S., Yakali E., Sharp J. J., O'Brien J. C., Berman P. W., Harbury H. A. Semisynthetic cytochrome c. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4248–4250. doi: 10.1073/pnas.74.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. A., Leaf G. The action of cyanogen bromide on horse-heart cytochrome c and horse-heart myoglobin. Biochem J. 1965 Sep;96(3):693–699. doi: 10.1042/bj0960693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBNALL A. C., MANGAN J. L., REES M. W. Studies on the amide and C-terminal residues in proteins. 3. The esterification of proteins. Biochem J. 1958 Jan;68(1):114–118. doi: 10.1042/bj0680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: reformation of the peptide bond linking residues 65 and 66. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1400–1406. doi: 10.1016/s0006-291x(74)80439-0. [DOI] [PubMed] [Google Scholar]
- Dyckes D. F., Creighton T., Sheppard R. C. Spontaneous re-formation of a broken peptide chain. Nature. 1974 Jan 25;247(5438):202–204. doi: 10.1038/247202a0. [DOI] [PubMed] [Google Scholar]
- Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971 Sep;124(3):581–590. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G. A., Jones J. H. A list of amino-acid derivatives which are useful in peptide synthesis. Int J Pept Protein Res. 1972;4(5):347–371. doi: 10.1111/j.1399-3011.1972.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Fletcher G. A., Jones J. H. A supplementary list of amino-acid derivatives which are useful in peptide synthesis. Int J Pept Protein Res. 1975;7(1):91–102. doi: 10.1111/j.1399-3011.1975.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez G., Offord R. E. The subunit structure of prealbumin. Biochem J. 1971 Nov;125(1):309–317. doi: 10.1042/bj1250309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. E., Offord R. E. A functioning complex between tryptic fragments of cytochrome c. A route to the production of semisynthetic analogues. Biochem J. 1977 Jan 1;161(1):21–25. doi: 10.1042/bj1610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E., Storey H. T., Rees A. R., Hayward C. F., Johnson W. H., Pheasey M. H., Wightman D. A. Diazoalkanes in peptide semisynthesis. Biochem J. 1976 Dec 1;159(3):480–486. doi: 10.1042/bj1590480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. The possible use of cyanogen bromide fragments in the semisynthesis of proteins and polypeptides. Biochem J. 1972 Sep;129(2):499–501. doi: 10.1042/bj1290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A. R., Offord R. E. The preparation of protected fragments of lysozyme for semisynthesis. Biochem J. 1976 Dec 1;159(3):467–479. doi: 10.1042/bj1590467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A. R., Offord R. E. The semisynthesis of portions of hen's-egg lysozyme by fragment condensation. Biochem J. 1976 Dec 1;159(3):487–493. doi: 10.1042/bj1590487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D. J., Offord R. Semisynthetic analogues of insulin. The use of N-substituted derivatives of methionine as acid-stable protecting groups. Biochem J. 1977 Sep 1;165(3):479–486. doi: 10.1042/bj1650479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotboom A. J., de Haas G. H. Specific transformations at the N-terminal region of phospholipase A2. Biochemistry. 1975 Dec 16;14(25):5394–5399. doi: 10.1021/bi00696a002. [DOI] [PubMed] [Google Scholar]
- Webster D., Offord R. E. The modification and removal of the N-terminal residue of trypsin by transamination. Biochem J. 1972 Nov;130(1):315–317. doi: 10.1042/bj1300315. [DOI] [PMC free article] [PubMed] [Google Scholar]


