Abstract
Intracellular serine proteinase was isolated from sporulating cells of Bacillus subtilis Marburg 168 by gramicidin S-Sepharose 4B affinity chromatography. The enzymological characteristics, the amino acid composition and the 19 residues of the N-terminal sequence of the enzyme are reported. The isolated proteinase was closely related to, but not completely identical with, the intracellular serine proteinase of B. subtilis A-50. The divergence between these two intracellular enzymes was less than that between the corresponding extracellular serine proteinases (subtilisins) of types Carlsberg and BPN', produced by these bacterial strains. This may be connected with the more strict selection constraints imposed in intracellular enzymes during evolution.
Full text
PDF

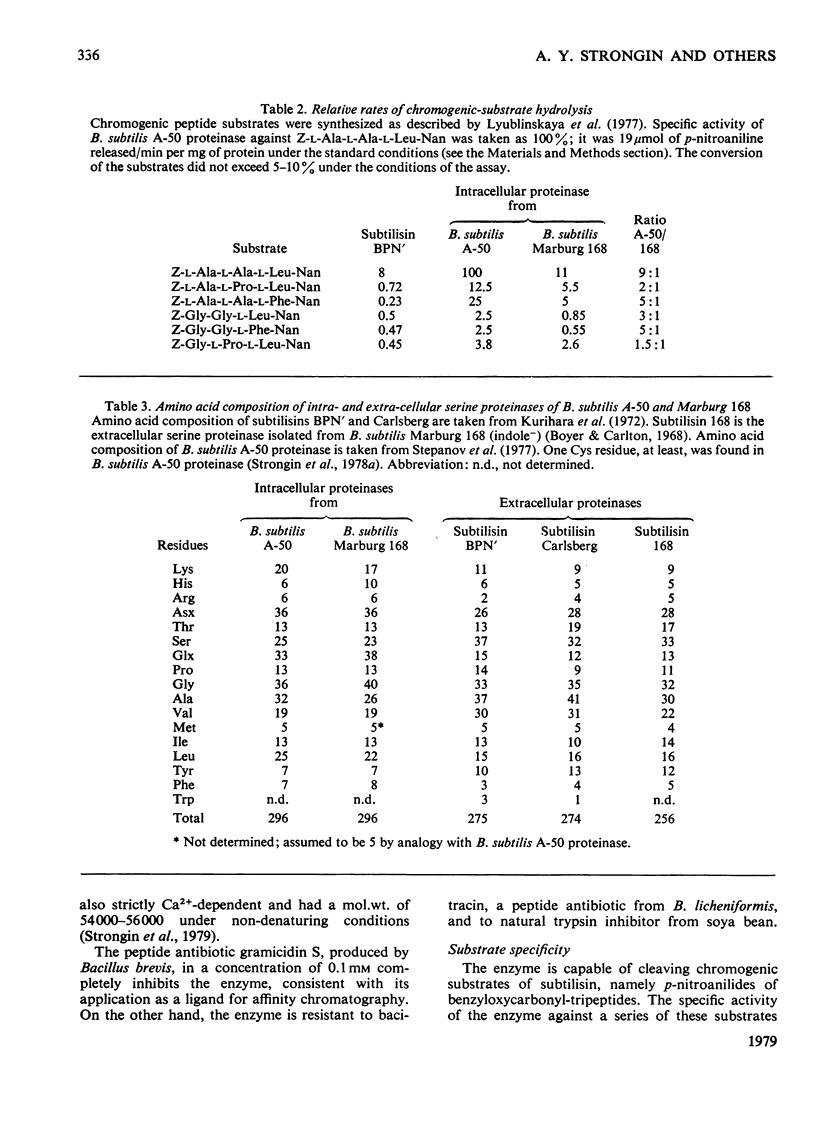
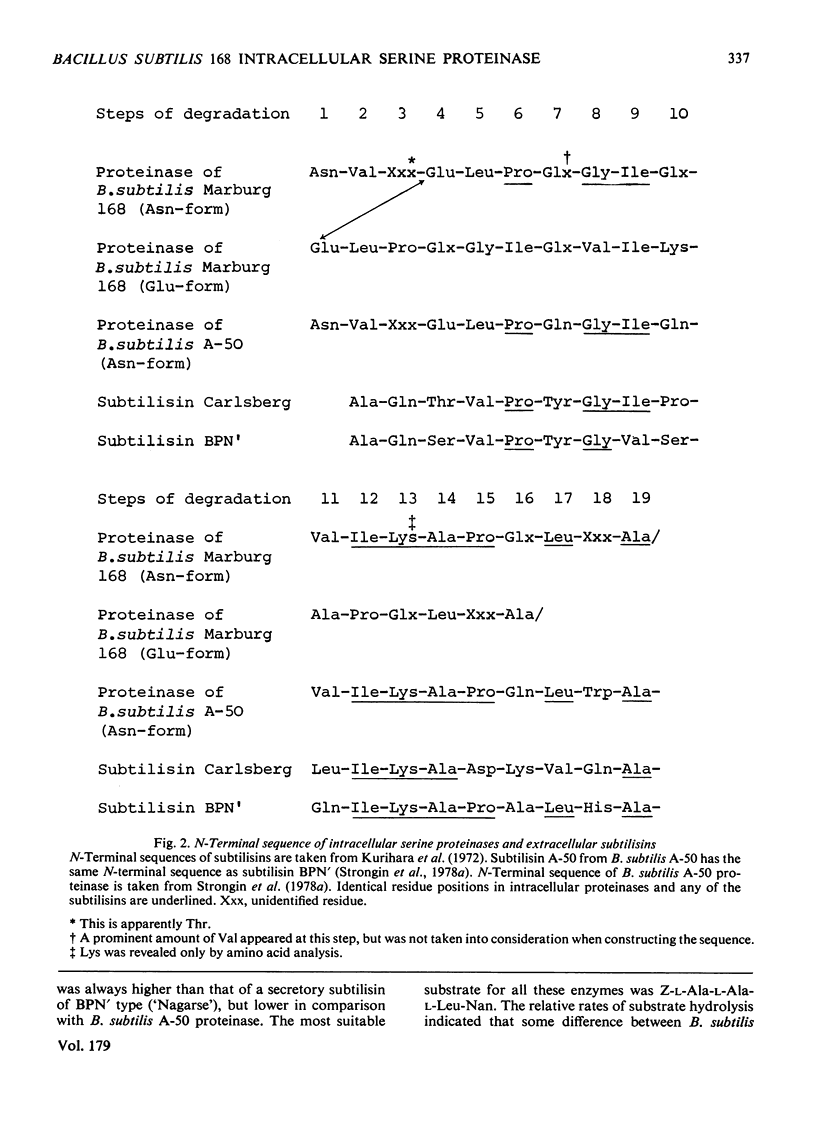


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Characterization and function of intracellular proteases in sporulating Bacillus cereus. Arch Microbiol. 1977 Oct 24;115(1):61–66. doi: 10.1007/BF00427846. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kurihara M., Markland F. S., Smith E. L. Subtilisin Amylosacchariticus. 3. Isolation and sequence of the chymotryptic peptides and the complete amino acid sequence. J Biol Chem. 1972 Sep 10;247(17):5619–5631. [PubMed] [Google Scholar]
- Lecadet M. M., Lescourret M., Klier A. Characterization of an intracellular protease isolated from Bacillus thuringiensis sporulating cells and able to modify homologous RNA polymerase. Eur J Biochem. 1977 Oct 3;79(2):329–338. doi: 10.1111/j.1432-1033.1977.tb11813.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification of protease II from Escherichia coli by affinity chromatography and separation of two enzyme species from cells harvested at late log phase. Eur J Biochem. 1976 Apr 15;64(1):199–204. doi: 10.1111/j.1432-1033.1976.tb10288.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Sibilli S., Bras G. Protease I from Escherichia coli. Some physicochemical properties and substrate specificity. Eur J Biochem. 1976 Oct 1;69(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10867.x. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Strongin A. Y., Izotova L. S., Abramov Z. T., Lyublinskaya L. A., Ermakova L. M., Baratova L. A., Belyanova L. P. Intracellular serine protease from Bacillus subtilis. Structural comparison with extracellular serine proteases-subtilisins. Biochem Biophys Res Commun. 1977 Jul 11;77(1):298–305. doi: 10.1016/s0006-291x(77)80196-4. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Ermakova L. M., Gorodetsky D. I., Stepanov V. M. On the appearance of Bacillus subtilis intracellular serine protease in the cell membrane and culture medium. Comparison of the enzyme and other Bacillus subtilis serine proteases. Arch Microbiol. 1978 Dec 20;119(3):287–293. doi: 10.1007/BF00405408. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Ermakova L. M., Baratova L. A., Belyanova L. P., Stepanov V. M. Intracellular serine protease of Bacillus subtilis: sequence homology with extracellular subtilisins. J Bacteriol. 1978 Mar;133(3):1401–1411. doi: 10.1128/jb.133.3.1401-1411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Stepanov V. M. Two related structural genes coding two homologous serine proteases in the Bacillus subtilis genome. Mol Gen Genet. 1978 Feb 27;159(3):337–339. doi: 10.1007/BF00268271. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]