Abstract
A high-molecular-weight glycoprotein (LGP-I) was shown [Swann, Sotman, Dixon & Brooks (1977) Biochem. J. 161, 473--485] to be the major constituent in the articular lubricating fraction from bovine synovial fluid. In addition to the LGP-I component, a second glycoprotein (LGP-II) was also present. After fractionation of bovine synovial fluid by sequential sedimentation in CsCl density gradients, the LGP-I and LGP-II components were separated by gel-permeation chromatography. The LGP-II component was then purified by chromatography on DEAE Bio-Gel A and Bio-Gel P-150. The molecular weight of the LGP-II component was 48,800 calculated from sedimentation-equilibrium measurements. Amino acids represented 53% (w/w) and carbohydrate constituents 36% (w/w) of the molecule. Glutamic acid and lysine (144 and 100 residues/1000 residues) were the major amino acids. Glucosamine, mannose, galactose and N-acetylneuraminic acid [representing 8.0, 6.6, 9.5 and 11.9% (w/w) respectively] were the only carbohydrate constituents detected. Immunodiffusion analysis showed that LGP-II component did not form a detectable precipitin line with antiserum to bovine serum. It appears likely, therefore, that this glycoprotein is synthesized by the joint tissues and is not derived from serum.
Full text
PDF

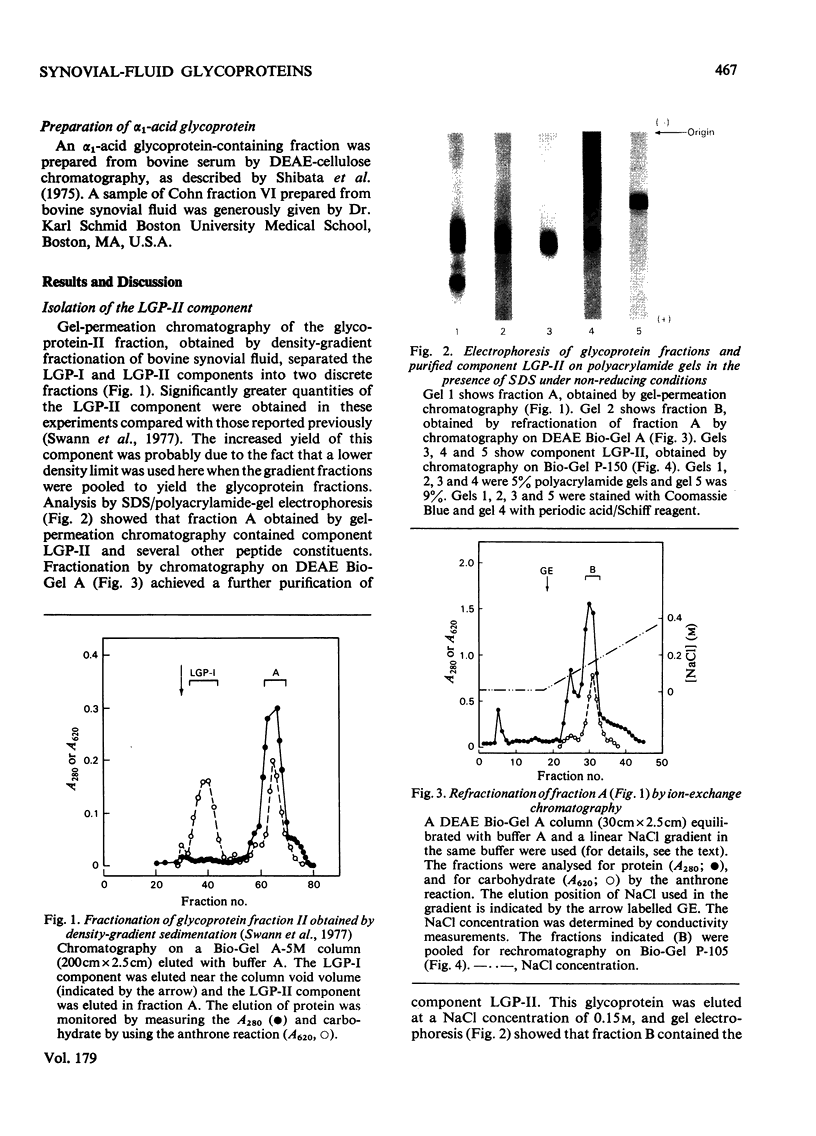
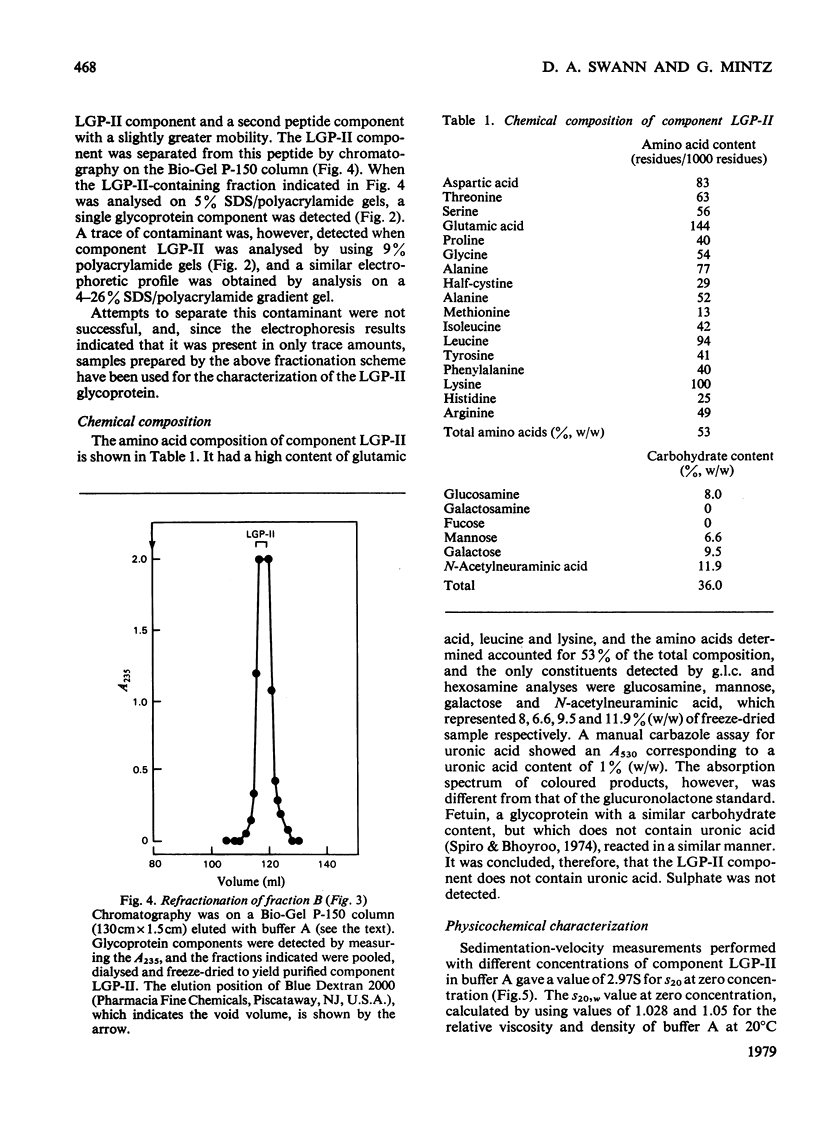
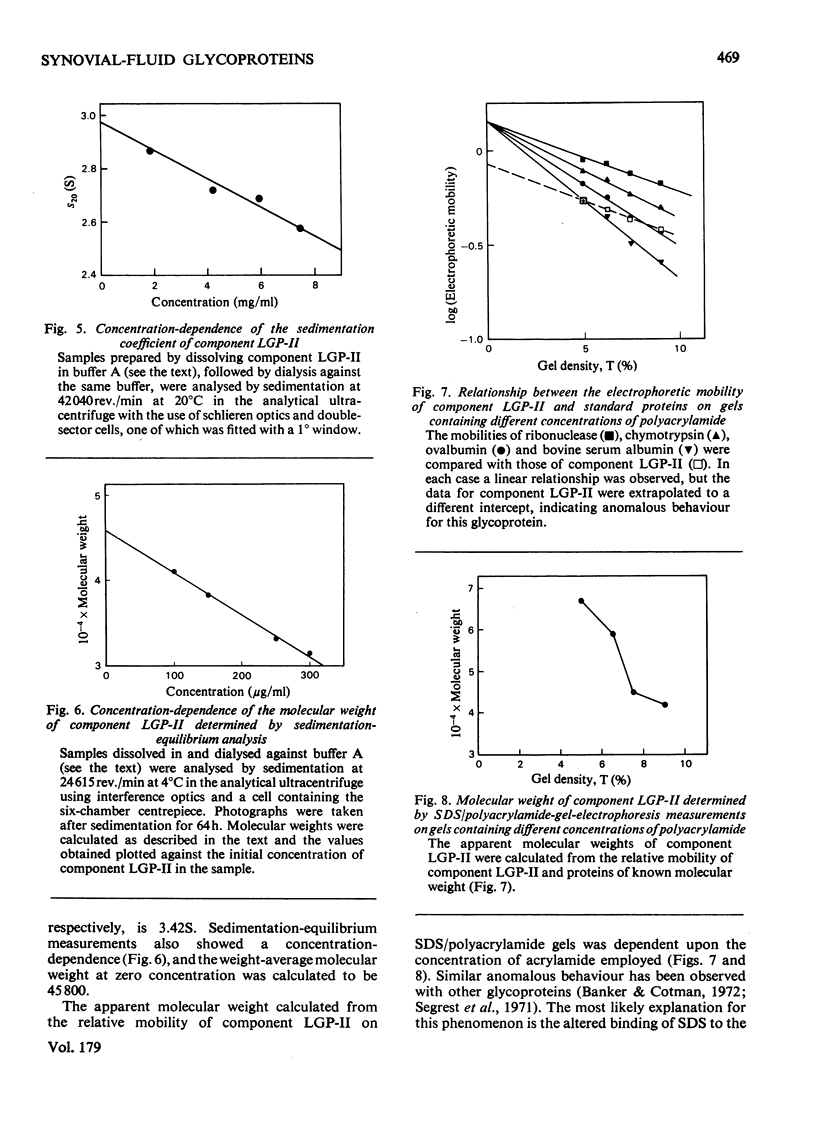


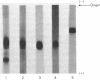
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEZKOROVAINY A., DOHERTY D. G. Isolation and characterization of an acidic glycoprotein from normal bovine plasma. Arch Biochem Biophys. 1962 Mar;96:491–499. doi: 10.1016/0003-9861(62)90326-0. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Endresen C., Christensen T. B. A comparison of the carbohydrate composition of serumorosomucoid from man, cattle, horse, and sheep. Acta Chem Scand. 1972;26(4):1743–1744. doi: 10.3891/acta.chem.scand.26-1743. [DOI] [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P., Rochu D., Fine J. M. A new method for determination of molecular weights of proteins by electrophoresis across a sodium dodecyl sulfate (SDS)-polyacrylamide gradient gel. Anal Biochem. 1976 Aug;74(2):567–575. doi: 10.1016/0003-2697(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Gilden R. V., Oroszlan S. Envelope glycoproteins of Rauscher murine leukemia virus: isolation and chemical characterization. Biochemistry. 1977 Feb 22;16(4):710–717. doi: 10.1021/bi00623a024. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Radin E. L., Swann D. A., Weisser P. A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970 Oct 24;228(5269):377–378. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Shibata K., Okubo H., Ishibashi H., Kawamura K. Human alpha1-acid glycoprotein: purification from urine proteins and immunological estimation of its serum concentration. Biochem Med. 1975 Jul;13(3):251–260. doi: 10.1016/0006-2944(75)90082-4. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Swann D. A., Radin E. L. The molecular basis of articular lubrication. I. Purification and properties of a lubricating fraction from bovine synovial fluid. J Biol Chem. 1972 Dec 25;247(24):8069–8073. [PubMed] [Google Scholar]
- Swann D. A., Sotman S., Dixon M., Brooks C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem J. 1977 Mar 1;161(3):473–485. doi: 10.1042/bj1610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]