Abstract
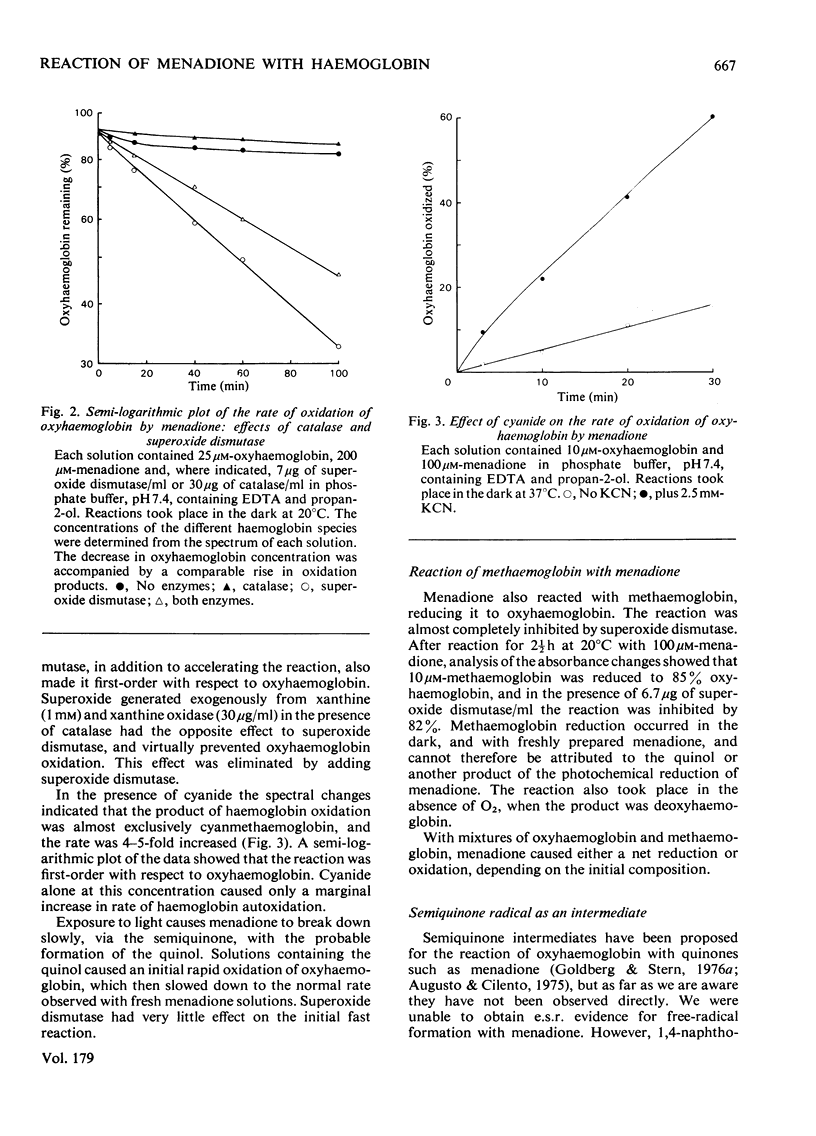
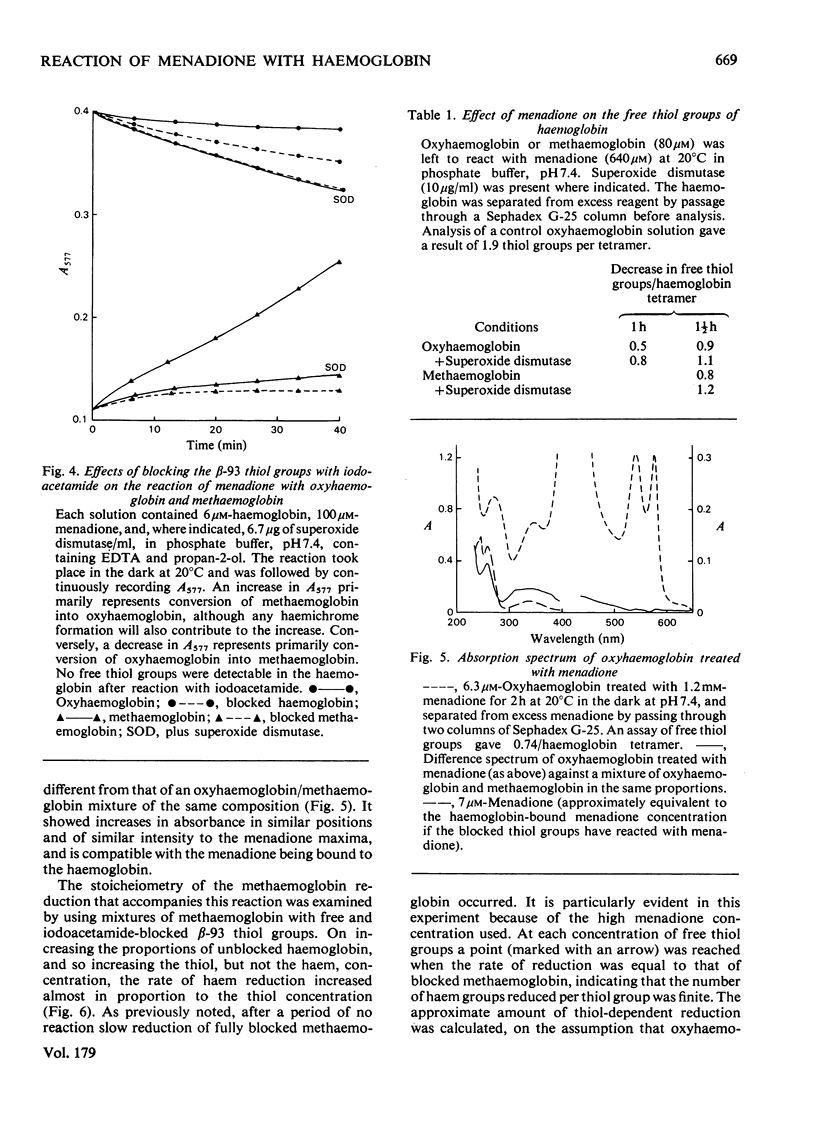
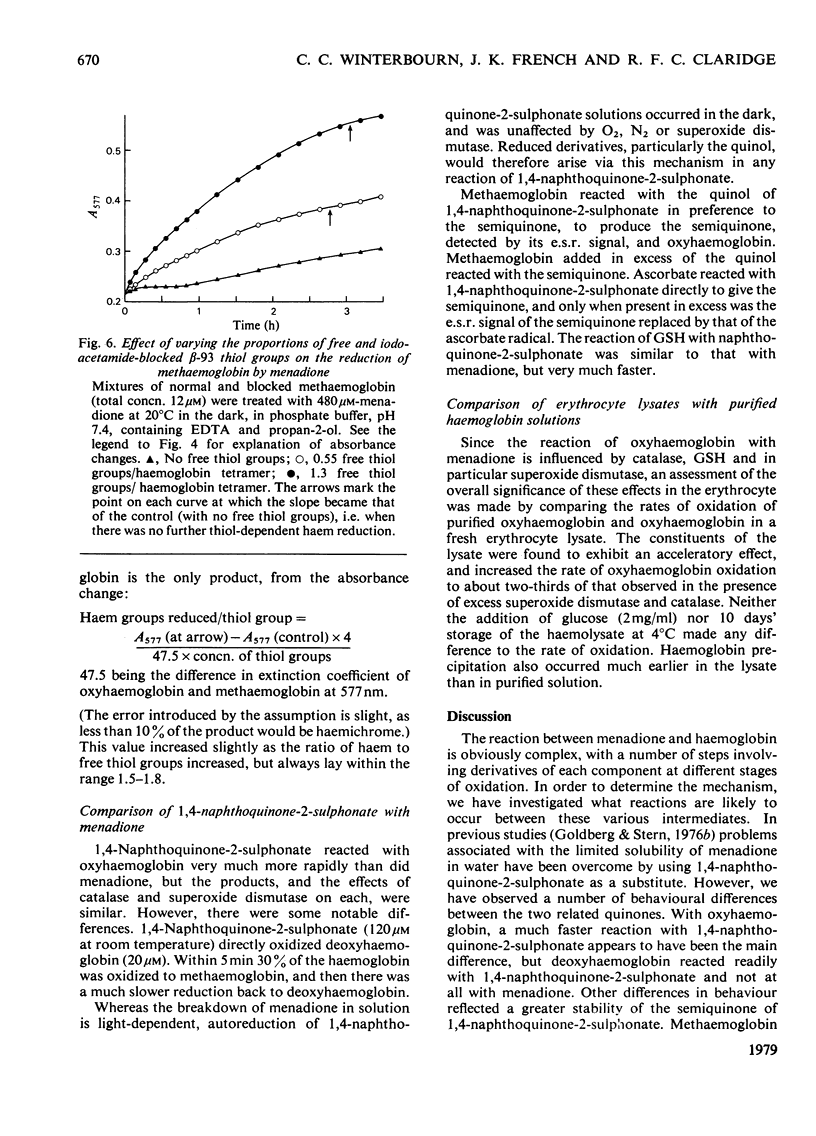
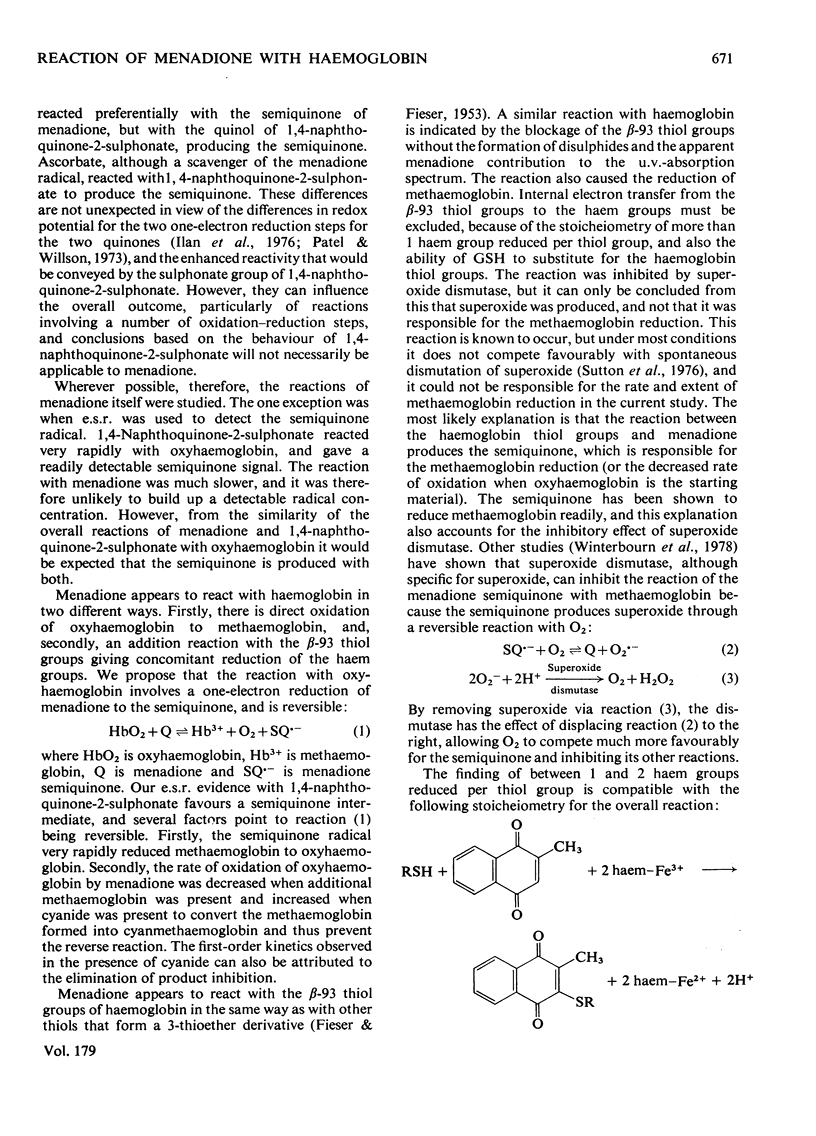
1. Menadione was found to react with both the haem groups and the beta-93 thiol groups of haemoglobin. 2. It oxidized the haem groups of oxyhaemoglobin, giving mainly methaemoglobin and a smaller amount of haemichrome. The reaction rate was decrease in the presence of catalase and markedly accelerated in the presence of superoxide dismutase. It is proposed that the overall reaction involves the initial reversible formation of methaemoglobin and the semiquinone, and that the effect of superoxide dismutase is to prevent the reverse reaction, by removing superoxide and hene O2-. E.s.r. evidence for the information of the semi-quinone and its reactions is presented. 3. The reaction of menadione with the beta-93 thiol groups of haemoglobin appeared to be similar to that with other thiols, forming the 3-thioether derivative of menadione, but it was also accompanied by reduction of methaemoglobin. This reduction was prevented by superoxide dismutase, but appeared to be caused by the semiquinone radical, which was produced as an intermediate. 4. Reduced glutathione functioned only to a limited extent as a scavenger of the menadione semiquinone. Its main reaction was directly with menadione to form the thioether. Ascorbate was a more efficient scavenger, and accelerated the oxidation of oxyhaemoglobin by menadione. 5. The significance of these findings in relation to menadione-induced erythrocyte haemolysis is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusto O., Cilento G. The effect of diphenols upon the autoxiation of oxyhemoglobin and oxymyoglobin. Arch Biochem Biophys. 1975 Jun;168(2):549–556. doi: 10.1016/0003-9861(75)90286-6. [DOI] [PubMed] [Google Scholar]
- Beutler E. Abnormalities of the hexose monophosphate shunt. Semin Hematol. 1971 Oct;8(4):311–347. [PubMed] [Google Scholar]
- Brown S. B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem J. 1976 Oct 1;159(1):23–27. doi: 10.1042/bj1590023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J. K., Winterbourn C. C., Carrell R. W. Mechanism of oxyhaemoglobin breakdown on reaction with acetylphenylhydrazine. Biochem J. 1978 Jul 1;173(1):19–26. doi: 10.1042/bj1730019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Stern A. Production of superoxide anion during the oxidation of hemoglobin by menadione. Biochim Biophys Acta. 1976 Jul 21;437(2):628–632. doi: 10.1016/0304-4165(76)90029-5. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Stern A. Superoxide anion as a mediator of drug-induced oxidative hemolysis. J Biol Chem. 1976 Oct 25;251(20):6468–6470. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The generation of O2-by the interaction of the hemolytic agent, phenylhydrazine, with human hemoglobin. J Biol Chem. 1975 Mar 25;250(6):2401–2403. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The mechanism of oxidative hemolysis produced by phenylhydrazine. Mol Pharmacol. 1977 Sep;13(5):832–839. [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr The use of 2,2'-dithiobis-(5-nitropyridine) as a selective reagent for the detection of thiols. J Chromatogr. 1969 Apr 22;41(1):121–123. doi: 10.1016/0021-9673(64)80109-6. [DOI] [PubMed] [Google Scholar]
- Ilan Y. A., Czapski G., Meisel D. The one-electron transfer redox potentials of free radicals. I. The oxygen/superoxide system. Biochim Biophys Acta. 1976 May 14;430(2):209–224. doi: 10.1016/0005-2728(76)90080-3. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- Mezick J. A., Settlemire C. T., Brierley G. P., Barefield K. P., Jensen W. N., Cornwell D. G. Erythrocyte membrane interactions with menadione and the mechanism of menadione-induced hemolysis. Biochim Biophys Acta. 1970 Dec 1;219(2):361–371. doi: 10.1016/0005-2736(70)90213-0. [DOI] [PubMed] [Google Scholar]
- Nakai N., Hase J. The reaction of 2-methyl-1,4-naphthoquinone with bovine serum albumin and papain. Chem Pharm Bull (Tokyo) 1968 Dec;16(12):2339–2342. doi: 10.1248/cpb.16.2339. [DOI] [PubMed] [Google Scholar]
- Rapp U., Adams W. C., Miller R. W. Purification of superoxide dismutase from fungi and characterization of the reaction of the enzyme with catechols by electron spin resonance spectroscopy. Can J Biochem. 1973 Feb;51(2):158–171. doi: 10.1139/o73-021. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J Clin Invest. 1974 Sep;54(3):678–689. doi: 10.1172/JCI107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., French J. K., Claridge R. F. Superoxide dismutase as an inhibitor of reactions of semiquinone radicals. FEBS Lett. 1978 Oct 15;94(2):269–272. doi: 10.1016/0014-5793(78)80953-3. [DOI] [PubMed] [Google Scholar]


