Abstract
The human FP-R (F2α prostaglandin receptor) is a Gq-coupled heptahelical ectoreceptor, which is of significant medical interest, since it is a potential target for the treatment of glaucoma and preterm labour. On agonist exposure, it mediates an increase in intracellular inositol phosphate formation. Little is known about the structures that govern the agonist-dependent receptor activation. In other prostanoid receptors, the C-terminal domain has been inferred in the control of agonist-dependent receptor activation. A DRY motif at the beginning of the second intracellular loop is highly conserved throughout the G-protein-coupled receptor family and appears to be crucial for controlling agonist-dependent receptor activation. It is replaced by an ERC motif in the FP-R and no evidence for the relevance of this motif in ligand-dependent activation of prostanoid receptors has been provided so far. The aim of the present study was to elucidate the potential role of the C-terminal domain and the ERC motif in agonist-controlled intracellular signalling in FP-R mutants generated by site-directed mutagenesis. It was found that substitution of the acidic Glu132 in the ERC motif by a threonine residue led to full constitutive activation, whereas truncation of the receptor's C-terminal domain led to partial constitutive activation of all three intracellular signal pathways that had previously been shown to be activated by the FP-R, i.e. inositol trisphosphate formation, focal adhesion kinase activation and T-cell factor signalling. Inositol trisphosphate formation and focal adhesion kinase phosphorylation were further enhanced by ligand binding in cells expressing the truncation mutant but not the E132T (Glu132→Thr) mutant. Thus C-terminal truncation appeared to result in a receptor with partial constitutive activation, whereas substitution of Glu132 by threonine apparently resulted in a receptor with full constitutive activity.
Keywords: constitutively active mutant, ERC motif, G-protein-coupled receptor, prostaglandin F2α receptor, site-directed mutagenesis, structure–function relationship
Abbreviations: FAK, focal adhesion kinase; GPCR, G-protein-coupled receptor; hFP-R, human F2α prostaglandin receptor; Lef, lymphoid enhancer factor; PG, prostaglandin; Tcf, T-cell factor
INTRODUCTION
The hFP-R [human F2α PG (prostaglandin) receptor] is one of the eight subtypes of prostanoid receptors that belong to the class of rhodopsin-like G-protein-coupled ectoreceptors with seven transmembrane domains [1,2]. It binds PGF2α and, with approx. 3-fold lower affinity, PGD2, both of which may be considered to be physiological ligands [3]. The affinity of the receptor for other prostanoids is lower by approximately two to three orders of magnitude. The receptor couples with Gq, thereby activating phospholipase C and increasing intracellular inositol trisphosphate and Ca2+ levels [3]. In addition, the FP-R has been shown to activate Rho-dependent signal cascades [4], presumably through an interaction with G12/13. Two C-terminal splice variants have been described for the ovine and bovine FP-R [5,6] that differ in their signal transduction properties; however, these C-terminal splice variants have been observed in no other species. The FP-R is of great clinical interest. Knockout mice lacking the FP-R are defective in the delivery of their pups [7,8]. Similarly, although through a different mechanism, the FP-R is of central physiological importance in the control of labour in women and attempts are being made to develop drugs that interfere with PGF2α signalling as a therapeutic approach to prevent preterm labour [9]. In addition, FP-R agonists have been devised for the treatment of glaucoma owing to the FP-R-dependent increases in uveoscleral outflow [10].
To date, only little is known about the structures of prostanoid receptors that govern the agonist-dependent control of downstream intracellular signalling. In previous studies, a conserved aspartate residue in transmembrane domain 7 of the FP-R or the EP3-R [11–13] and a phenylalanine residue in the second intracellular loop of the TP-R [14] have been implicated in ligand-dependent control of G-protein activation. Studies with splice variants of the EP3-R [15–19] as well as truncation mutants of the EP3-R [15,16] and studies with hybrid receptors in which the EP3-R C-terminal domain was replaced by C-terminal domains of other prostanoid receptors [20,21] imply a function of the C-terminal domain of prostanoid receptors in agonist-dependent coupling control. For many other GPCRs (G-protein-coupled receptors), the proximal part of the second intracellular loop has been shown to be crucial in agonist-dependent control of G-protein activation (see [22] and references therein). A DRY motif is highly conserved in this region in most GPCRs. Substitution of the arginine residue in this conserved motif resulted in mutants with decreased efficiency in ligand-dependent G-protein activation [23], whereas replacement of the aspartate residue in this motif with any other amino acid resulted in receptor mutants with different degrees of constitutive agonist-independent activity [24], the mutation to D142T (Asp142→Thr) being the most active. The function of the conserved tyrosine residue was not systematically analysed. Notably, the sequence motif DRY is changed to an ERC motif in most prostanoid receptors including the human FP-R [3]. There are no studies so far that address the question of whether this ERC motif in prostanoid receptors fulfils the same function as the DRY motif in other GPCRs.
It was therefore the purpose of the present study to analyse a possible contribution of the C-terminal domain and the ERC motif to the agonist-dependent and -independent activation of the downstream intracellular signal chain by the human FP-R. To this end, mutations were generated by site-directed mutagenesis in which the C-terminal domain was truncated after 10 amino acids distal to the putative end of transmembrane domain 7 at a site that corresponds to the natural splice site of the ovine FP-R [5]. In additional mutants, amino acids were substituted individually or in combination in the ERC motif. It was found that truncation of the C-terminal domain and substitution of glutamate residue by a threonine residue in the ERC motif yielded receptors with partial or full constitutive activity, implying a role of both the C-terminal domain and the second intracellular loop in the control of agonist-dependent FP-R activation.
EXPERIMENTAL
Materials
All materials were of analytical grade and from commercial sources indicated in the text. Oligonucleotides were custom synthesized (MWG-Biotech, Ebersberg, Germany).
PCR-based site-directed mutagenesis
The FLAG–hFP cDNA, which had previously been amplified from human placenta [11] and had been cloned into pcDNA3, served as template for PCR-based site-directed mutagenesis. Mutations were generated using Power script polymerase (PAN Biotech, Aidenbach, Germany) according to the following programme: 3 min 95 °C, 35 times (1 min 95 °C, 1 min 55 °C and 2 min 72 °C) and 10 min 72 °C. The following primer combinations were used hFP318Stop-R: hFP318Stop-f (CTCTATAAGCTTTAGAGTCAATGC) and hFP-r (cggcgtctagaCTAGGTGCTTGCTGATTTCTCTGC). The PCR-product was digested with HindIII (recognition sequence contained in the forward sequence) and XbaI (recognition site contained in the reverse primer shown underlined). The resulting fragment was cloned into FLAG-hFP-pcDNA3 and digested with the same enzymes. The mutants hFP-E132D-R, hFP-C134Y-R and hFP-E132D/C134Y-R were generated using a degenerate primer: 5′-fragment T7-primer (TAATACGACTCACTATAGGGAGA), hFP-ERC/DRY-r [CCAATA(C/T)ACCG(G/C)TCAATGGCCATCACAC]; 3′-fragment hFP-R-ERC-DRY-f [GTGTGATGGCCATTGA(G/C)CGGT(A/G)TATTGG] and hFP-r. The 5′- and 3′-receptor fragments were used as mega primers in a PCR including the flanking primers, T7-primer and hFP-r, to generate the cDNA containing the entire translated region of the mutated FLAG-tagged hFP-Rs (where hFP-R stands for human FP-R). The final cDNA constructs were cloned into the XhoI–XbaI site of pcDNA3 and identified by sequencing. hFP-E132T-R: 5′-fragment T7-primer, hFP-E132T-r (CCGCGTAATGGCCATCACAC); 3′-fragment hFP-E132T-f (GTGTGATGGCCATTACGCGG), hFP-r. The fragments were combined, cloned and characterized as above. The cDNAs for the wild-type receptor and all receptor mutants were finally subcloned into the KpnI–ApaI site of pcDNA5/FRT. The final products were sequenced to confirm the successful introduction of the mutations and to exclude additional mutations due to PCR artefacts. All sequencing reactions were performed by automated sequencing employing a Beckman Coulter capillary DNA sequencer CEQ 2000 XL with the corresponding sequencing kit provided by the company and universal flanking vector primers.
Expression of the mutant receptor proteins
HEK-293-FlpIn cells (Invitrogen, Groningen, The Netherlands; where HEK-293 stands for human embryonic kidney 293) were cultured in Dulbecco's modified Eagle's medium containing 5% (v/v) fetal calf serum, 1% penicillin/streptomycin and 100 μg/ml zeocin. They were transfected with the respective hFP-R constructs at the DNA concentration indicated by the modified calcium phosphate procedure described previously [25]. After transfection, the culture was continued for 24 h without zeocin. Unless otherwise indicated, experiments were started 24 h after transfection. For the integration of expression plasmids into the genome, cells were co-transfected with the respective hFP-R constructs and a 10-fold excess of the FlpIn-recombinase plasmid. After transfection, the culture was continued for 24 h without zeocin and then continued under selection with hygromycin.
Membrane isolation and PGF2α binding assay
Membrane preparation and binding assays were performed essentially as described previously [25,26]. For membrane preparations, cells expressing the wild-type or mutant receptor proteins were scraped into a homogenization buffer containing 50 mM Tris/HCl (pH 7.5), 5 mM EDTA and 0.2 mM Pefabloc SC (Biomol, Hamburg, Germany), 10 μg/ml leupeptin and 10 μg/ml soya-bean trypsin inhibitor as protease inhibitors. After homogenization by vigorous pipetting and vortex-mixing, a crude membrane fraction was prepared by centrifugation of the homogenate at 100000 g. The resulting pellet was suspended in binding buffer containing 10 mM Mes/NaOH (pH 6.2), 10 mM MgCl2 and 1 mM EDTA and stored at −70 °C. Protein was determined by a method described by Bradford [27]. For saturation binding assays, 150 μg of protein membranes were incubated with 0.5–5 nM [3H]PGF2α (6.7 TBq/mmol) and increasing concentrations of unlabelled PGF2α in 100 μl of binding buffer for 1 h at 20 °C. Non-specific binding was determined in the presence of 10 μM PGF2α. Bound and unbound ligands were separated by rapid vacuum filtration through GF 52 filters (Schleicher and Schuell, Dassel, Germany). Filters were washed five times with 4 ml of ice-cold binding buffer. Radioactivity retained on the filter was counted in 5 ml of Rotiszint (Roth, Karlsruhe, Germany). Binding constants were calculated by non-linear regression analysis (LIGAND) [28].
Cell surface ligand binding
Transfected cells in 24-well plates (5×104 cells/well) were washed once with incubation buffer (Hepes-buffered salt solution, pH 7.4, containing 15 mM Hepes, 4.7 mM KCl, 140 mM NaCl, 1.2 mM KH2PO4, 11 mM glucose and 2.2 mM CaCl2) and then incubated for 2 h at 4 °C with 100 μl of 5 nM [3H]PGF2α. An excess of unlabelled PGF2α (10 μM) was added into some of the wells to determine non-specific binding. Plates were washed three times with ice-cold incubation buffer and cell-associated radioactivity was released by lysing cells in 400 μl of 0.3 M NaOH and 1% (w/v) SDS. The radioactivity in the cell lysates was counted in 5 ml of Rotiszint 22.
Determination of PGF2α-stimulated inositol phosphate formation
HEK-293-FlpIn cells transiently transfected with cDNAs for FP-R mutants, 24 h before the experiment, were labelled by replacing the culture medium with inositol-free Dulbecco's modified Eagle's medium containing 10% (v/v) dialysed fetal bovine serum and 1 μCi/ml myo-[3H]inositol. After 24 h, free inositol was removed by extensive washing. Cells were then incubated with Krebs–Henseleit buffer (110 mM NaCl, 5.5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 20 mM NaHCO3 and 11 mM glucose) containing 10 mM LiCl to inhibit degradation of inositol phosphates. PGF2α was added to the cells to a final concentration of 1 μM where indicated. After 15 min, the reaction was stopped by removing the buffer and freezing the cells in liquid N2. Cells were scraped into 750 μl of 10 mM formic acid. The homogenate was neutralized with 3 ml of 10 mM ammonia and loaded on to 1.5 ml of DOWEX (1×8, 200–400 mesh) formate columns. Columns were washed with 4 ml of 0.04 M ammonium formate/0.04 M formic acid (pH 5.0) and inositol phosphates were eluted with 4 ml of 2 M ammonium formate/2 M formic acid (pH 5.0). Radioactivity in aliquots of the fractions was determined using Rotiszint scintillant.
Determination of FAK (focal adhesion kinase) phosphorylation
Cells that had been selected by hygromycin for integration of the vector containing the respective receptor cDNAs were seeded on 3.5 cm poly-L-lysine-coated tissue culture plates at a density of 3×105 cells. Cells were stimulated with 1 μM PGF2α for 5 min, 24 h after seeding. The medium was removed, cells were washed once with PBS and then homogenized in 800 μl of lysis buffer [1% (v/v) Triton X-100, 0.05% SDS, 50 mM Hepes (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 2 mM Pefabloc SC, 10 μg/ml leupeptin and 10 μg/ml soya-bean trypsin inhibitor]. The homogenate was centrifuged at 4 °C for 30 min at 35000 g. The supernatant was preabsorbed for 1 h at 4 °C with 100 μl of 10% (v/v) Sepharose 4B in lysis buffer containing 1% (w/v) BSA. Sepharose was removed by centrifugation and the supernatant was incubated with 1 μg of anti-FAK antibody (Santa Cruz Biotechnology, Heidelberg, Germany) at 4 °C overnight. Immune complexes were then precipitated by incubation with 100 μl of Protein G–Sepharose in lysis buffer containing 1% BSA for 1 h at 4 °C. The precipitate was washed twice with wash buffer (10 mM Pipes, pH 7.0, and 100 mM NaCl) containing 0.5% (v/v) Nonidet P40 and once with wash buffer without Nonidet P40. The final precipitate was incubated in Laemmli SDS sample buffer for 5 min at 95 °C. Proteins were separated by PAGE and blotted on to PVDF membranes. After adding blocking buffer [5% (w/v) skimmed milk in 20 mM Tris/HCl (pH 7.0), 138 mM NaCl and 0.1% (v/v) Tween 20], proteins were detected first with a phosphotyrosine-specific primary antibody (mAK 4G10; Upstate Biotechnology, Lake Placid, NY, U.S.A.) and again detected after stripping with an FAK-specific antibody (Santa Cruz Biotechnology), the appropriate peroxidase-coupled secondary antibodies and subsequent detection by chemiluminescence according to the instructions of the manufacturer (Pierce, Rockford, IL, U.S.A.).
Tcf (T-cell factor)/Lef (lymphoid enhancer factor) reporter gene assay
FP-R-dependent activation of the Tcf/Lef pathway was determined in a reporter gene assay essentially as described by Fujino [29]. Briefly, cells were co-transfected with the cDNA of the FP-R-mutant of interest, pCMV-β-Gal and either the luciferase reporter gene construct (pTOPFLASH) or a negative control (pFOP-FLASH). The medium was replaced by fresh medium ±1 μM PGF2α, 24 h after transfection. After 24 h, cells were washed twice with PBS and stored in liquid N2. Cells were lysed in 100 μl of lysis buffer (Promega, Mannheim, Germany) and luciferase was determined according to the manufacturer's instructions (Promega). As a control for transfection efficiency, β-galactosidase activity was determined photometrically at 405 nm in 1 μl of the same lysates transferred into 100 μl of ONPG buffer (100 mM sodium phosphate, pH 7.5, 13 mM MgSO4 and 6 mM 2-nitrophenyl-β-galactopyranoside).
RESULTS
Ligand binding properties of wild-type and mutant FP-Rs
Crude membranes were prepared from HEK-293-FlpIn cells that had been selected by hygromycin treatment for the expression of the wild-type FP-R and the mutant receptors. Saturation binding assays were performed with these membranes. Wild-type and mutant receptors bound to PGF2α with an apparent Kd of approx. 10 nM (Table 1). No significant difference in the Kd values was observed between the wild-type and the mutant receptors. The level of receptor expression in membranes of transiently transfected cells varied between 1 and 5 pmol/mg of membrane protein (Table 1) when cells were transfected with 10 μg of DNA/106 cells.
Table 1. Binding properties of wild-type and mutant human FP-Rs.
Kd values were determined by non-linear regression analysis of saturation binding assays with membranes of cells expressing the respective receptor construct. The maximum specific binding capacity Bmax was calculated from specific binding at a single concentration (5 nM) taking into account the Kd value. Values are means±S.E.M. for at least three independent experiments. WT, wild-type.
| Receptor | Kd (nM) | Bmax (fmol/mg) |
|---|---|---|
| hFP WT | 13.4±6.0 | 2560±366 |
| hFP 318Stop | 13.4±2.9 | 1130±89 |
| hFP E132D | 9.8±1.6 | 5450±1010 |
| hFP C134Y | 13.7±3.0 | 1942±521 |
| hFP E132D,C134Y | 9.5±1.7 | 4211±709 |
| hFP E132T | 14.2±4.7 | 2563±283 |
Agonist-induced and constitutive second messenger formation in cells expressing wild-type or mutant FP-Rs
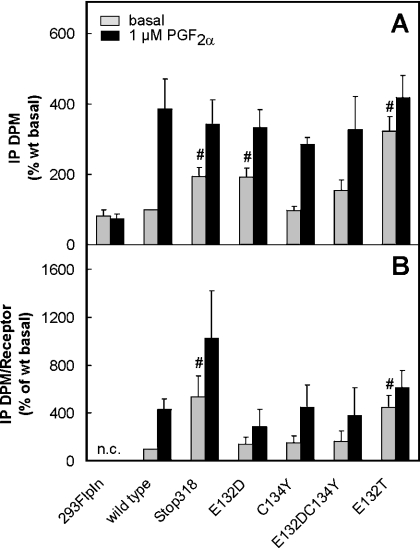
Cells transiently transfected with the cDNAs for wild-type or mutant FP-Rs were labelled with myo-[3H]inositol. Basal and agonist-induced inositol phosphate accumulation was determined in these cells. Basal inositol phosphate formation in wild-type receptor-expressing cells was set at 100% in each experiment to account for potential differences in labelling efficiency between the experiments. Basal inositol phosphate formation was marginally higher in wild-type receptor-expressing cells when compared with the mock-transfected controls (2229±783 d.p.m./106 cells versus 1870±831 d.p.m./106 cells, means±S.D.). This difference, however, was not statistically significant. On stimulation with 1 μM PGF2α, inositol phosphate formation did not change in mock-transfected cells but increased by 4–5-fold above the basal value in cells expressing the wild-type FP-R (Figure 1A). Inositol phosphate formation elicited by saturating concentrations of the agonist was similar in cells expressing wild-type or mutant receptors, indicating that all receptors coupled efficiently with the downstream signal chain. However, basal inositol phosphate formation was significantly higher in cells expressing either the truncated receptor hFP-318Stop-R or receptors in which Glu132 had been replaced either by aspartate (hFP-E132D-R) or a threonine residue (hFP-E132T-R) when compared with mock-transfected or wild-type receptor-transfected cells. The agonist-independent inositol phosphate formation was most pronounced in cells expressing the hFP-E132T-R. In these cells, it reached almost the same extent as the agonist-induced inositol phosphate formation in cells expressing wild-type receptor. Substitution of Cys134 by tyrosine (hFP-C134Y-R) did not enhance agonist-independent basal inositol phosphate formation. Basal inositol phosphate formation was not elevated in cells expressing the double mutant E132D,C134Y, although the isolated substitution E132D appeared to increase agonist-independent inositol phosphate formation to some extent.
Figure 1. Basal and agonist-induced inositol phosphate formation in cells transiently transfected with cDNAs for wild-type and mutant hFP-R proteins.
Transiently transfected HEK-293-FlpIn cells were labelled with myo-[3H]inositol for 24 h. Inositol phosphate formation was determined in non-stimulated cells and cells exposed to 1 μM PGF2α for 15 min. Inositol phosphates were extracted with 10 mM formic acid. Radioactively labelled inositol phosphates (IP) were separated from other labelled compounds by ion exchange chromatography on DOWEX formate and quantified by liquid scintillation counting. To allow pooling of data of independent transfection experiments with varying labelling efficiency, inositol phosphate formation is expressed as percentage of the basal inositol phosphate formation in wild-type expressing (wt) cells in the same experiment (A). The number of [3H]PGF2α binding sites was determined in crude membranes of parallely transfected wells and the amount of inositol phosphate formed was corrected for the different expression levels of the receptor proteins (B). The values are means±S.E.M. for 3–7 independent experiments. n.c., not calculated. Statistics: Student's t test for unpaired samples; #, significantly larger than wild-type control P<0.05.
The disadvantage of transient transfection is the variability in transfection efficiency between different experiments, which lead to variations in receptor expression levels between experiments, even if the plasmid concentration for transfection is kept constant. Ligand binding was therefore determined in membranes of plates transfected in parallel in all individual experiments. This allowed a correction of basal and agonist-induced inositol phosphate formation for receptor density in each individual experiment. After correction for the receptor density, basal inositol phosphate formation remained significantly higher in cells expressing the FP-R truncated receptor and the mutant receptor, in which Glu132 had been replaced by a threonine residue, indicating that these receptors might be constitutively active. In addition, agonist-stimulated inositol phosphate formation in cells expressing the C-terminally truncated mutant appeared to be higher than in wild-type-transfected cells (Figure 1B).
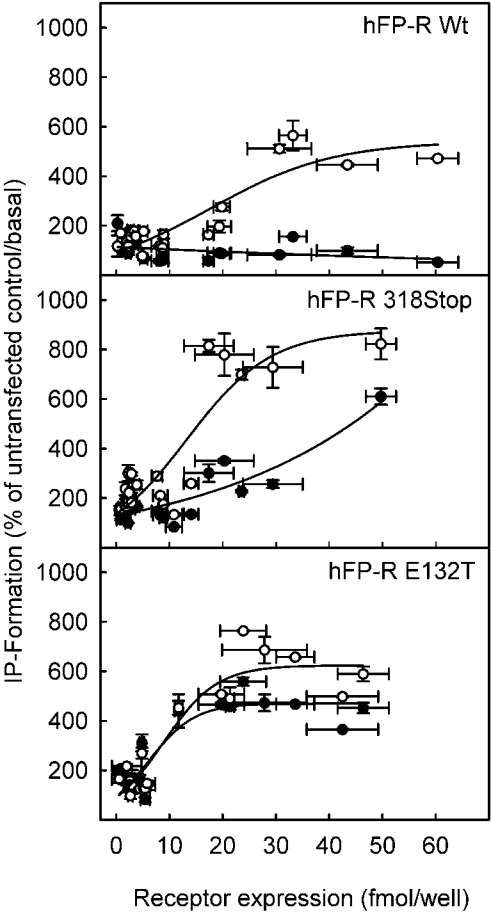
To corroborate this finding further, the dependence of basal and agonist-induced inositol phosphate formation on the receptor density was analysed systematically for the wild-type and the two receptor mutants with putative constitutive activity, the hFP-318Stop-R and the hFP-E132T-R (Figure 2). Cells were transfected with varying concentrations of cDNAs ranging from 0.5 to 25 μg/106 cells for the respective receptors. The number of PGF2α binding sites was determined in triplicate for each individual transfection and each concentration of transfected cDNA. Binding to the surface of the intact cell instead of to the crude membrane preparations was determined in these experiments since only cell surface receptors are functionally relevant. In parallel wells, the basal and agonist-induced inositol phosphate formation was determined in these cells. The inositol phosphate formation was then plotted as a function of the receptor density. At no receptor concentration was basal inositol phosphate formation different from untransfected controls in cells expressing the hFP-R wild-type. On stimulation with a 1 μM saturating concentration of the agonist, inositol phosphate formation increased as a function of receptor density, reaching a maximum of approx. 5-fold above basal values at approx. 30 fmol of receptor/well corresponding to approx. 120 fmol/106 cells. In contrast with cells transfected with the FP-R wild-type, basal inositol phosphate formation was significantly higher than in the mock-transfected controls in cells expressing the hFP-318Stop-R at receptor concentrations higher than 16 fmol/well. This agonist-independent inositol phosphate formation increased with increasing receptor concentrations, indicating that the receptor activated the downstream signal chain also when not occupied by the ligand. Notably, however, at any given receptor concentration, the basal inositol phosphate formation was further enhanced by saturating concentrations of the agonist, indicating that the receptor in the absence of ligand was not fully active towards the downstream signal chain. As with the wild-type receptor, the maximal activation of the downstream signalling chain by a receptor saturating concentration of agonist was achieved with a receptor density of 32 fmol of receptor/well. However, maximal inositol phosphate formation was 8-fold over basal values and not merely 5-fold over basal values as with the wild-type receptor, indicating that the coupling efficiency of the truncated receptor was higher. Similar to cells expressing the truncated receptor, cells expressing the E132T mutant receptor showed an agonist-independent inositol phosphate formation that increased with increasing receptor concentrations. However, in marked contrast with the truncated receptor in cells expressing the E132T mutant, this agonist-independent inositol phosphate formation was almost as high as in the presence of saturating concentrations of the agonist at any given receptor concentration. In summary, the wild-type receptor did not display basal constitutive activity, the stop-mutant appeared to be partially constitutively active but the receptor-dependent inositol phosphate formation was further enhanced by ligand binding, whereas the E132T mutant showed almost full constitutive activity.
Figure 2. Dependence of agonist-dependent and agonist-independent inositol phosphate formation on the receptor density.
HEK-293-FlpIn cells were transfected with increasing concentrations of the receptor cDNA indicated. After 24 h, cells were labelled with myo-[3H]inositol for 24 h. Inositol phosphate formation was determined in non-stimulated cells (●) and cells exposed to 1 μM PGF2α for 15 min (○). Inositol phosphates were separated from inositol by chromatography as described in the caption of Figure 1. Parallel plates were used to determine the receptor density by binding of [3H]PGF2α to intact cells. Unspecific binding was determined in the presence of 10 μM unlabelled PGF2α. Values are means±S.E.M. for triplicate determinations for at least three independent experiments.
Agonist-induced and constitutive activation of alternative FP-R intracellular signal pathways in cells expressing wild-type or mutant FP-Rs
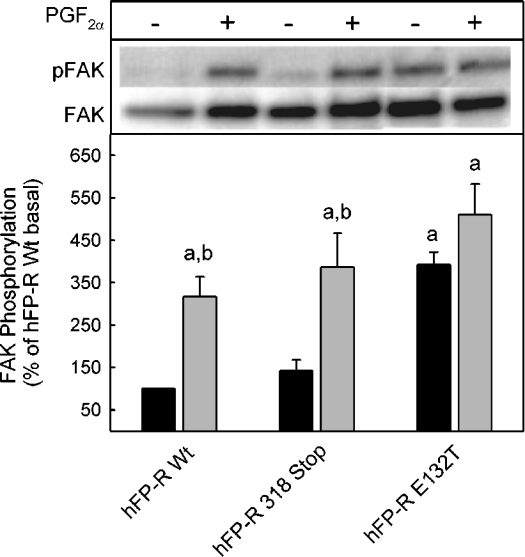
It has previously been shown that FP-Rs, in addition to enhancing inositol trisphosphate formation, can activate a Rho-dependent intracellular signal chain thereby increasing the phosphorylation of p125 FAK and inducing cell rounding [4]. Therefore the question was addressed whether the partial constitutive activity of the truncated receptor and the full constitutive activity also extend to the Rho-dependent signalling. Cells expressing the truncated receptor had a morphology and growth behaviour that was not different from wild-type receptor-expressing cells. In contrast, cells expressing the E132T mutants grew much more slowly and did not spread properly on the tissue culture plate but rather grew in small clusters and tended to detach from the culture plate (results not shown). The poor growth made it impossible to passage E132T mutant-expressing cells by more than a few generations, since cultures were overgrown by cells that had lost receptor expression. Basal phosphorylation of FAK was low in cells expressing the wild-type FP-R and was enhanced about 3-fold by PGF2α (Figure 3). In cells expressing the truncated FP-R basal FAK, phosphorylation was only marginally higher than in wild-type receptor-expressing cells and was increased to a similar extent by PGF2α. Consistent with the aberrant growth behaviour of cells expressing the E132T mutant receptor, in these cells FAK in the absence of PGF2α was phosphorylated to a similar extent as in wild-type receptor-expressing cells in the presence of the agonist. Phosphorylation was not further enhanced significantly by PGF2α in cells expressing the E132T mutant. In conclusion, this receptor mutant appeared to activate constitutively the Rho-dependent signalling also.
Figure 3. Constitutive activation of FAK in cells expressing the FP-E132T receptor.
Cells expressing wild-type FP-R or the indicated receptor mutant were incubated for 5 min in the presence or absence of 1 μM PGF2α, washed with PBS buffer and then lysed. The lysate was centrifuged and the supernatant was subjected to an immunoprecipitation with an FAK-specific antibody. Immunoprecipitated proteins were separated by SDS/PAGE and blotted on to PVDF membranes. Proteins were quantified with a phosphotyrosine-specific antibody (pFAK) and after stripping and restaining with an FAK-specific antibody, an appropriate peroxidase-labelled secondary antibody and a chemiluminescence substrate. Total (FAK) and phosphorylated (pFAK) immunoreactivities on the same blot are given. Values were normalized to the values of FAK and pFAK in the absence of PGF2α in cells expressing the wild-type (Wt) receptor. They are means±S.E.M. for three independent experiments. Statistics: Student's t test; a, significantly different from wild-type non-stimulated control; and b, significantly different from respective non-stimulated cells.
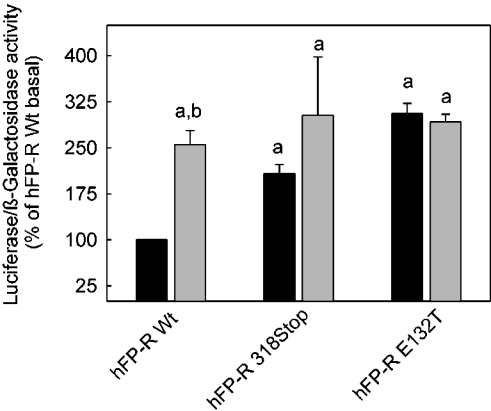
Evidence has been provided that an activation of Rho might lead to the release of β-catenin from cadherins and thereby activate Tcf-dependent transcription [30] and that PGF2α might increase transcription under the control of such a promoter by Rho activation [29,31]. Therefore the transcriptional activity from a Tcf/Lef promoter was analysed in cells co-transfected with a Tcf-luciferase reporter gene construct and either wild-type or mutant FP-Rs (Figure 4). PGF2α increased luciferase expression under the control of this promoter to approx. 3-fold in cells expressing the wild-type receptor. Agonist-independent luciferase expression was 2-fold higher in cells expressing the truncated receptor. Luciferase expression was further enhanced by PGF2α to the same final activity as in wild-type receptor-expressing cells, although this difference between basal and agonist-induced luciferase activity was not statistically significant in these cells (Figure 4). Basal luciferase activity in cells expressing the E132T mutant receptor was already as high as in wild-type receptor-expressing cells in the presence of PGF2α and was not further enhanced by the agonist. In summary, these results indicate that a partial or full agonist-independent activation of downstream signal chains by the truncated and E132T FP-R mutant respectively was not restricted to the activation of the Gq-dependent signalling but extended also to the presumably G12/13- and Rho-dependent signalling.
Figure 4. Constitutive activation of the transcription of a luciferase reporter gene from a Tcf-controlled promoter in cells expressing either the truncated hFP-R318 Stop mutant or the hFP-R E132T mutant.
Cells were co-transfected with plasmids containing the cDNA for the receptor mutant indicated, either a plasmid pTOPFLASH containing the luciferase gene under the control of Tcf/Lef to monitor the β-catenin/Tcf/Lef-dependent activation of transcription or the plasmid pFOPFLASH, in which the Tcf-site was mutated, as a negative control and a plasmid containing the β-galactosidase gene under a cytomegalovirus promoter to monitor transfection efficiency. Cells were exposed to medium with (grey bars) or without (black bars) 1 μM PGF2α, 24 h after transfection, and cultured for an additional 24 h. Cells were lysed. Luciferase and β-galactosidase activities were determined with appropriate assays in cell lysates. Values were calculated as follows: luciferase activity of pFOPFLASH-transfected cells was subtracted from the activity in the corresponding pTOPFLASH-transfected cells. The ratio between this pTOPFLASH-specific activity and the β-galactosidase activity was determined. The ratio obtained with wild-type (Wt) FP-R-expressing cells in the absence of PGF2α was set at 100% for every experiment. Values are means±S.E.M. for three independent experiments. Statistics: Student's t test; a, significantly different from wild-type non-stimulated control; and b, significantly different from respective non-stimulated cells.
Receptor internalization
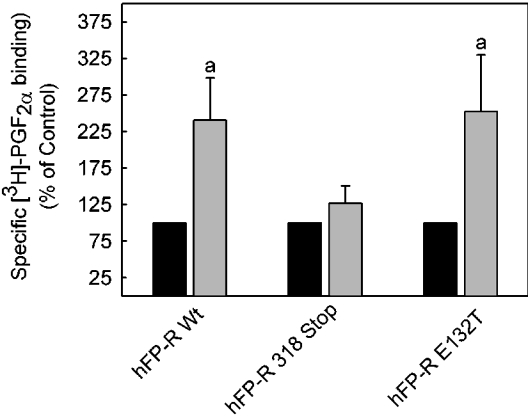
The FP-R has been shown to undergo a presumably protein kinase C-dependent desensitization and internalization, both in cellular systems that naturally express the receptor [32] and after heterologous expression [33,34]. It was therefore assumed that the constitutively active receptor mutant might be internalized in the absence of an agonist to a greater extent than the wild-type receptor. To test this hypothesis, clathrin-dependent internalization of the receptor was suppressed by co-transfection of an haemagglutinin-tagged GTPase-deficient K44A dominant negative dynamin [35]. Surprisingly, the number of cell surface accessible binding sites was increased approx. 2-fold by dominant negative dynamin in cells expressing either wild-type or the fully constitutively active E132T mutant receptor (Figure 5), whereas cell surface receptor expression was not affected in cells expressing the truncated receptor mutant. These results show that, at variance with the expectations, the constitutively active mutant receptor was not internalized to a larger extent than the wild-type receptor in the absence of a ligand. The results also support previous findings that the C-terminal domain of the FP-R is essential for clathrin-mediated receptor internalization [34].
Figure 5. Increase in cell surface accessible PGF2α binding sites by co-expression of dominant negative dynamin K44A with wild-type receptor and FP-R 132T mutant but not with the C-terminally truncated FP-R 318 Stop mutant.
Cells were co-transfected with a plasmid containing the cDNA for the FP-R wild-type or the receptor mutant indicated and either an expression plasmid containing the cDNA for an haemagglutinin-tagged dominant negative dynamin K44A (grey bars) or green fluorescent protein under the same promoter (black bars). Cell surface binding of [3H]PGF2α was determined at 4 °C, 48 h after transfection, as described in the Experimental section. Specific binding in cells co-transfected with the green fluorescent protein plasmid was set at 100% in each case. Values are means±S.E.M. for three independent experiments. Statistics: Student's t test; a, significantly different from control.
DISCUSSION
The highly conserved DRY motif at the beginning of the second intracellular loop, which is replaced by an ERC motif in the human FP-R and most other prostanoid receptors, as well as the C-terminal domain have been implicated in agonist-dependent G-protein coupling control in many GPCRs. In line with this general principle, mutants of the human FP-R in which either the acidic residue of the ERC motif was replaced by a threonine residue or the C-terminal domain was truncated had enhanced constitutive activity (Figures 1–4).
Dependence of agonist-induced and agonist-independent inositol phosphate formation in hFP wild-type receptor-expressing cells on the receptor density
According to the widely accepted model, GPCRs exist in at least two different conformational states: an inactive state, which precludes the activation of the G-protein, and an active state, which allows the activation of the G-protein. Ligand binding shifts the equilibrium from the inactive state to the active state [36]. The extent of G-protein activation and hence the rate of second messenger formation depends on the amount of receptor in the active state. At receptor saturating concentrations of the ligand or with constitutively active receptors in the absence of the ligand, the rate of second messenger formation should thus increase with increasing receptor numbers within a certain range of receptor concentrations. In line with this model, a linear dependence on receptor density of basal agonist-independent inositol phosphate formation has previously been described for the angiotensin II type 1A receptor [37]. For the wild-type FP-R, a dependence of second messenger formation on receptor density in the presence of saturating agonist concentrations was also observed at a calculated receptor concentration between approx. 20 and 120 fmol/106 cells (5–30 fmol/well, Figure 2). Below a concentration of 10 fmol/106 cells, there was no detectable increase in inositol phosphate formation at receptor-saturating PGF2α concentrations, whereas an increase in the FP-R concentration above 120 fmol/106 cells did not result in a further increase in agonist-dependent inositol phosphate formation (Figure 2). In theory, any GPCR should activate the downstream signalling chain also in the absence of a ligand if expressed at sufficiently high levels to allow an increase in the absolute number of receptor molecules in the active state above the threshold value needed to elicit a detectable change in second messenger formation. With the wild-type FP-R, a ligand-independent second messenger formation was not observed even if the receptor levels were 2-fold higher than those required for maximal agonist-induced second messenger formation. This indicates that the equilibrium between the inactive and active state in the wild-type FP-R, in contrast with e.g. the angiotensin II type 1A receptor [37], is far on the side of the inactive state.
Function of the ERC motif
There is a detailed hypothesis regarding how the DRY motif in GPCRs might control receptor activation. This hypothesis is supported by molecular modelling and characterization of mutant receptors [36]. According to this hypothesis, the acidic residue of the DRY motif is essential to keep the receptor in the inactive state. It prevents the positively charged arginine residue in the motif from forming other interactions that will directly or indirectly lead to the activation of the G-protein. The acidic residue may interact either directly with the arginine residue or stabilize a polar pocket formed by transmembrane domains 1, 2 and 7 that buries the arginine residue. Current findings with the FP-R suggest that the ERC motif might play a similar role in this receptor. Substitution with a threonine residue, which in the human α1B-adrenergic receptor led to maximal constitutive activity [24], resulted in an agonist-independent inositol phosphate formation (Figure 1B) that increased with the amount of receptor expressed in transfected cells (Figure 2). The receptor appeared to be almost fully constitutively active since at any given receptor concentration, inositol phosphate formation was not significantly enhanced by the agonist (Figures 1B and 2). The curves of second messenger formation as a function of receptor density in the presence and absence of an agonist were almost congruent. Thus the equilibrium between the receptor in the active and inactive conformation appeared to be shifted almost completely to the active conformation by substitution of Glu132 with the threonine residue. The receptor concentration needed for half-maximal second messenger formation and the maximal agonist-induced second messenger formation were similar to the values determined for the wild-type receptor. Hence, the coupling efficiency of the receptor apparently was not affected by the substitution of Glu132 with a threonine residue.
Conservative substitution of the glutamic residue by aspartic residue had only a questionable effect on basal inositol phosphate formation. The absolute increase in agonist-independent inositol phosphate formation, which was observed in cells transfected with this receptor (Figure 1A), can probably be attributed to the higher average expression level in these cells since no difference between wild-type and mutant receptor was observed when inositol phosphate formation was corrected for receptor density (Figure 1B). In addition, there was no increase in basal inositol phosphate formation in cells expressing the double mutant, in which Glu132 had been replaced by an aspartic residue and Cys134 had been replaced by a tyrosine residue. The possibility that the receptor is kept in the inactive state only by either the combination Glu132 and Cys134 or the combination Asp132 and Tyr134 appears to be unlikely, since the isolated substitution of Cys134 by tyrosine neither increased the basal level nor interfered with the agonist-induced inositol phosphate formation. However, the observation that the wild-type receptor did not confer agonist-independent inositol phosphate formation over a wide range of receptor concentrations (Figure 2), even exceeding the average receptor concentration of the experiments in Figure 1, might indicate that, compared with the wild-type receptor, substitution of Glu132 by an aspartic residue led to a slight shift in the equilibrium between the different receptor conformations towards the active state.
Apparently, the conformational change supposedly caused by the substitution of Glu132 by a threonine residue resulted not only in the activation of the Gq-dependent downstream signal chain but also conferred activation of the probably G12/13- and Rho-dependent signalling, resulting in a phosphorylation of FAK (Figure 3) and an increase in reporter gene transcription under the control of a Tcf-dependent promoter (Figure 4).
Function of the C-terminal domain
Agonist-independent inositol phosphate formation was increased in cells expressing high levels of the FP-R mutant lacking the C-terminal domain (Figures 1 and 2). At all receptor concentrations, inositol phosphate formation was further increased by saturating concentrations of the agonist. To achieve a similar increase in second messenger formation, higher concentrations of the receptor were needed in the absence of an agonist than in its presence. Hence, truncation led to a receptor with partially constitutive activity. Little is known regarding how the C-terminal domain might contribute to agonist-induced receptor activation. It was assumed that, in prostanoid receptors, it sterically hinders the interaction of the inactive receptor with the G-proteins [15]. The idea that the receptor's C-terminal domain interferes with the receptor G-protein interaction in a rather unspecific mode is also supported by the finding that the degree of constitutive activity in C-terminal splice variants of the EP3-R in different species directly correlates with the length of the C-terminal domain rather than with conserved sequence motifs [15]. In addition, the constitutive activity that was caused by a truncation of the EP3-R [16] was abolished by substitution of the EP3-R C-terminal domain with the structurally completely unrelated but larger domains of the human EP4-R [21] or I-type PG receptor [20]. Thus the C-terminal domain might not contain structural motifs that are involved in switching from inactive to active forms of receptors or are needed to restrain the receptor in its inactive conformation as discussed in [38], but rather the C-terminal domain of prostanoid receptors might interfere in the interaction with the G-protein of both the inactive and the active receptors as also proposed for the EP3 receptor [16]. In line with such a hypothesis, maximal agonist-induced inositol phosphate formation for each receptor appeared to be higher in cells expressing the C-terminally truncated mutant when compared with the cells expressing the wild-type FP-R (Figures 1B and 2). At variance with such a hypothesis, half-maximal inositol phosphate formation at saturating ligand concentrations was observed at similar concentrations of the wild-type receptor and the C-terminally truncated receptor. The observation that C-terminal truncation enhanced G-protein coupling efficiency also contrasts with metabotropic glutamate receptors in which the C-terminal domain appears to confer more efficient coupling with the G-protein [39], and reduced coupling is observed in splice variants with shorter C-terminal domains.
In addition to its role in coupling control, the C-terminal domain of prostanoid receptors has been implicated in clathrin-dependent receptor internalization. Thus different splice variants of the EP3-R differ in their agonist-induced desensitization [40] and internalization. Similarly, the C-terminal domain of the EP4-R is necessary and sufficient to confer agonist-induced phosphorylation, desensitization and clathrin-dependent internalization [25,41]. Of the two ovine FP-R splice variants, only the one with the full-length C-terminal domain was internalized in a clathrin-dependent manner [31,34]. In line with the general concept that the C-terminal domain of the prostanoid receptor is essential for clathrin-dependent internalization, in the present study, it was shown that the cell surface expression of the truncated FP-R mutant was not affected by interruption of the clathrin-dependent internalization with dominant negative dynamin K44A (Figure 5), whereas the cell surface expression of wild-type receptor and E132T mutant receptor, which both contain the full-length C-terminal domain, was enhanced by the co-expression of dominant negative dynamin K44A.
Acknowledgments
The technical assistance of M. Kuna is gratefully acknowledged. This study was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB366, Teilprojekt A16).
References
- 1.Sakamoto K., Kamimura M., Kurozumi S., Ito S. Prostaglandin F2 alpha receptor. J. Lipid Mediat. Cell Signal. 1995;12:405–411. doi: 10.1016/0929-7855(95)00026-m. [DOI] [PubMed] [Google Scholar]
- 2.Narumiya S., Sugimoto Y., Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 3.Abramovitz M., Boie Y., Nguyen T., Rushmore T. H., Bayne M. A., Metters K. M., Slipetz D. M., Grygorczyk R. Cloning and expression of a cDNA for the human prostanoid FP receptor. J. Biol. Chem. 1994;269:2632–2636. [PubMed] [Google Scholar]
- 4.Pierce K. L., Fujino H., Srinivasan D., Regan J. W. Activation of FP prostanoid receptor isoforms leads to Rho-mediated changes in cell morphology and in the cell cytoskeleton. J. Biol. Chem. 1999;274:35944–35949. doi: 10.1074/jbc.274.50.35944. [DOI] [PubMed] [Google Scholar]
- 5.Pierce K. L., Bailey T. J., Hoyer P. B., Gil D. W., Woodward D. F., Regan J. W. Cloning of a carboxyl-terminal isoform of the prostanoid FP receptor. J. Biol. Chem. 1997;272:883–887. doi: 10.1074/jbc.272.2.883. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto K., Ishii Y., Onodera T., Sugano T. Cloning and characterization of the novel isoforms for PGF2 alpha receptor in the bovine corpus luteum. DNA Seq. 2002;13:307–311. doi: 10.1080/1042517021000011645. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto Y., Segi E., Tsuboi K., Ichikawa A., Narumiya S. Female reproduction in mice lacking the prostaglandin F receptor. Roles of prostaglandin and oxytocin receptors in parturition. Adv. Exp. Med. Biol. 1998;449:317–321. doi: 10.1007/978-1-4615-4871-3_39. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto Y., Yamasaki A., Segi E., Tsuboi K., Aze Y., Nishimura T., Oida H., Yoshida N., Tanaka T., Katsuyama M., et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 9.Peri K. G., Quiniou C., Hou X., Abran D., Varma D. R., Lubell W. D., Chemtob S. THG113: a novel selective FP antagonist that delays preterm labor. Semin. Perinatol. 2002;26:389–397. doi: 10.1053/sper.2002.37307. [DOI] [PubMed] [Google Scholar]
- 10.Weinreb R. N., Toris C. B., Gabelt B. T., Lindsey J. D., Kaufman P. L. Effects of prostaglandins on the aqueous humor outflow pathways. Surv. Ophthalmol. 2002;47:S53–S64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 11.Neuschäfer-Rube F., Engemaier E., Koch S., Böer U., Püschel G. P. Identification by site-directed mutagenesis of amino acids contributing to ligand-binding specificity or signal transduction properties of the human FP prostanoid receptor. Biochem. J. 2003;371:443–449. doi: 10.1042/BJ20021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Audoly L., Breyer R. M. Substitution of charged amino acid residues in transmembrane regions 6 and 7 affect ligand binding and signal transduction of the prostaglandin EP3 receptor. Mol. Pharmacol. 1997;51:61–68. doi: 10.1124/mol.51.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Satoh S., Chang C., Katoh H., Hasegawa H., Nakamura K., Aoki J., Fujita H., Ichikawa A., Negishi M. The key amino acid residue of prostaglandin EP3 receptor for governing G-protein association and activation steps. Biochem. Biophys. Res. Commun. 1999;255:164–168. doi: 10.1006/bbrc.1998.0161. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H., Yan F., Yamamoto S., Tai H. H. Phenylalanine 138 in the second intracellular loop of human thromboxane receptor is critical for receptor-G-protein coupling. Biochem. Biophys. Res. Commun. 1999;264:171–175. doi: 10.1006/bbrc.1999.1508. [DOI] [PubMed] [Google Scholar]
- 15.Jin J., Mao G. F., Ashby B. Constitutive activity of human prostaglandin E receptor EP3 isoforms. Br. J. Pharmacol. 1997;121:317–323. doi: 10.1038/sj.bjp.0701121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa H., Negishi M., Ichikawa A. Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J. Biol. Chem. 1996;271:1857–1860. doi: 10.1074/jbc.271.4.1857. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa H., Negishi M., Katoh H., Ichikawa A. Two isoforms of prostaglandin EP3 receptor exhibiting constitutive activity and agonist-dependent activity in Rho-mediated stress fiber formation. Biochem. Biophys. Res. Commun. 1997;234:631–636. doi: 10.1006/bbrc.1997.6655. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa A., Negishi M., Hasegawa H. Three isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive Gi activity and agonist-dependent Gs activity. Adv. Exp. Med. Biol. 1997;433:239–242. doi: 10.1007/978-1-4899-1810-9_51. [DOI] [PubMed] [Google Scholar]
- 19.Irie A., Sugimoto Y., Namba T., Asano T., Ichikawa A., Negishi M. The C-terminus of the prostaglandin-E-receptor EP3 subtype is essential for activation of GTP-binding protein. Eur. J. Biochem. 1994;224:161–166. doi: 10.1111/j.1432-1033.1994.tb20007.x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Kirchrath J., Hasse A., Schrör K. Preservation of Gi coupling of a chimeric EP3/I-type prostaglandin (IP) receptor. Biochem. Pharmacol. 1999;58:471–476. doi: 10.1016/s0006-2952(99)00119-7. [DOI] [PubMed] [Google Scholar]
- 21.Neuschäfer-Rube F., Hänecke K., Blaschke V., Jungermann K., Püschel G. P. The C-terminal domain of the Gs-coupled EP4 receptor confers agonist-dependent coupling control to Gi but no coupling to Gs in a receptor hybrid with the Gi-coupled EP3 receptor. FEBS Lett. 1997;401:185–190. doi: 10.1016/s0014-5793(96)01468-8. [DOI] [PubMed] [Google Scholar]
- 22.Chung D. A., Wade S. M., Fowler C. B., Woods D. D., Abada P. B., Mosberg H. I., Neubig R. R. Mutagenesis and peptide analysis of the DRY motif in the alpha2A adrenergic receptor: evidence for alternate mechanisms in G-protein-coupled receptors. Biochem. Biophys. Res. Commun. 2002;293:1233–1241. doi: 10.1016/S0006-291X(02)00357-1. [DOI] [PubMed] [Google Scholar]
- 23.Scheer A., Costa T., Fanelli F., De Benedetti P. G., Mhaouty-Kodja S., Abuin L., Nenniger-Tosato M., Cotecchia S. Mutational analysis of the highly conserved arginine within the Glu/Asp-Arg-Tyr motif of the alpha(1b)-adrenergic receptor: effects on receptor isomerization and activation. Mol. Pharmacol. 2000;57:219–231. [PubMed] [Google Scholar]
- 24.Scheer A., Fanelli F., Costa T., De Benedetti P. G., Cotecchia S. The activation process of the alpha1B-adrenergic receptor: potential role of protonation and hydrophobicity of a highly conserved aspartate. Proc. Natl. Acad. Sci. U.S.A. 1997;94:808–813. doi: 10.1073/pnas.94.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuschäfer-Rube F., Oppermann M., Möller U., Böer U., Püschel G. P. Agonist-induced phosphorylation by G-protein-coupled receptor kinases of the EP4 receptor carboxyl-terminal domain in an EP3/EP4 prostaglandin E(2) receptor hybrid. Mol. Pharmacol. 1999;56:419–428. doi: 10.1124/mol.56.2.419. [DOI] [PubMed] [Google Scholar]
- 26.Neuschäfer-Rube F., DeVries C., Hänecke K., Jungermann K., Püschel G. P. Molecular cloning and expression of a prostaglandin E2 receptor of the EP3 beta subtype from rat hepatocytes. FEBS Lett. 1994;351:119–122. doi: 10.1016/0014-5793(94)00837-x. [DOI] [PubMed] [Google Scholar]
- 27.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 29.Fujino H., Regan J. W. FP prostanoid receptor activation of a T-cell factor/beta-catenin signaling pathway. J. Biol. Chem. 2001;276:12489–12492. doi: 10.1074/jbc.C100039200. [DOI] [PubMed] [Google Scholar]
- 30.Meigs T. E., Fields T. A., McKee D. D., Casey P. J. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta-catenin release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujino H., Srinivasan D., Regan J. W. Cellular conditioning and activation of beta-catenin signaling by the FPB prostanoid receptor. J. Biol. Chem. 2002;277:48786–48795. doi: 10.1074/jbc.M209393200. [DOI] [PubMed] [Google Scholar]
- 32.Püschel G. P., Miura H., Neuschäfer-Rube F., Jungermann K. Inhibition by the protein kinase C activator 4 beta-phorbol 12-myristate 13-acetate of the prostaglandin F2 alpha-mediated and noradrenaline-mediated but not glucagon-mediated activation of glycogenolysis in rat liver. Eur. J. Biochem. 1993;217:305–311. doi: 10.1111/j.1432-1033.1993.tb18247.x. [DOI] [PubMed] [Google Scholar]
- 33.Fujino H., Srinivasan D., Pierce K. L., Regan J. W. Differential regulation of prostaglandin F(2alpha) receptor isoforms by protein kinase C. Mol. Pharmacol. 2000;57:353–358. [PubMed] [Google Scholar]
- 34.Srinivasan D., Fujino H., Regan J. W. Differential internalization of the prostaglandin f(2alpha) receptor isoforms: role of protein kinase C and clathrin. J. Pharmacol. Exp. Ther. 2002;302:219–224. doi: 10.1124/jpet.302.1.219. [DOI] [PubMed] [Google Scholar]
- 35.Al-Hasani H., Hinck C. S., Cushman S. W. Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J. Biol. Chem. 1998;273:17504–17510. doi: 10.1074/jbc.273.28.17504. [DOI] [PubMed] [Google Scholar]
- 36.Parnot C., Miserey-Lenkei S., Bardin S., Corvol P., Clauser E. Lessons from constitutively active mutants of G-protein-coupled receptors. Trends Endocrinol. Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 37.Parnot C., Bardin S., Miserey-Lenkei S., Guedin D., Corvol P., Clauser E. Systematic identification of mutations that constitutively activate the angiotensin II type 1A receptor by screening a randomly mutated cDNA library with an original pharmacological bioassay. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7615–7620. doi: 10.1073/pnas.110142297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hizaki H., Hasegawa H., Katoh H., Negishi M., Ichikawa A. Functional role of carboxyl-terminal tail of prostaglandin EP3 receptor in Gi coupling. FEBS Lett. 1997;414:323–326. doi: 10.1016/s0014-5793(97)01020-x. [DOI] [PubMed] [Google Scholar]
- 39.Prezeau L., Gomeza J., Ahern S., Mary S., Galvez T., Bockaert J., Pin J. P. Changes in the carboxyl-terminal domain of metabotropic glutamate receptor 1 by alternative splicing generate receptors with differing agonist-independent activity. Mol. Pharmacol. 1996;49:422–429. [PubMed] [Google Scholar]
- 40.Negishi M., Sugimoto Y., Irie A., Narumiya S., Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J. Biol. Chem. 1993;268:9517–9521. [PubMed] [Google Scholar]
- 41.Neuschäfer-Rube F., Hermosilla R., Rehwald M., Rönnstrand L., Schülein R., Wernstedt C., Püschel G. P. Identification of a Ser/Thr cluster in the C-terminal domain of the human prostaglandin receptor EP4 that is essential for agonist-induced beta-arrestin1 recruitment but differs from the apparent principal phosphorylation site. Biochem. J. 2004;379:573–585. doi: 10.1042/BJ20031820. [DOI] [PMC free article] [PubMed] [Google Scholar]