Abstract
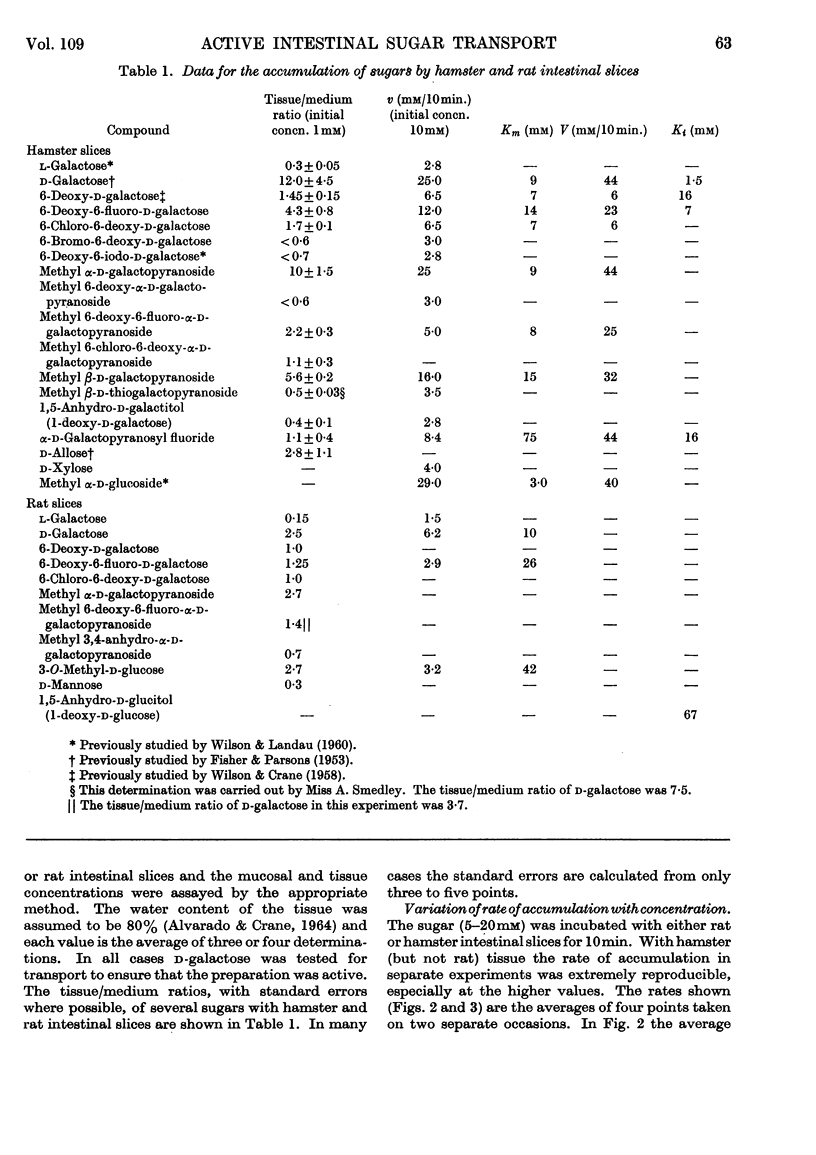
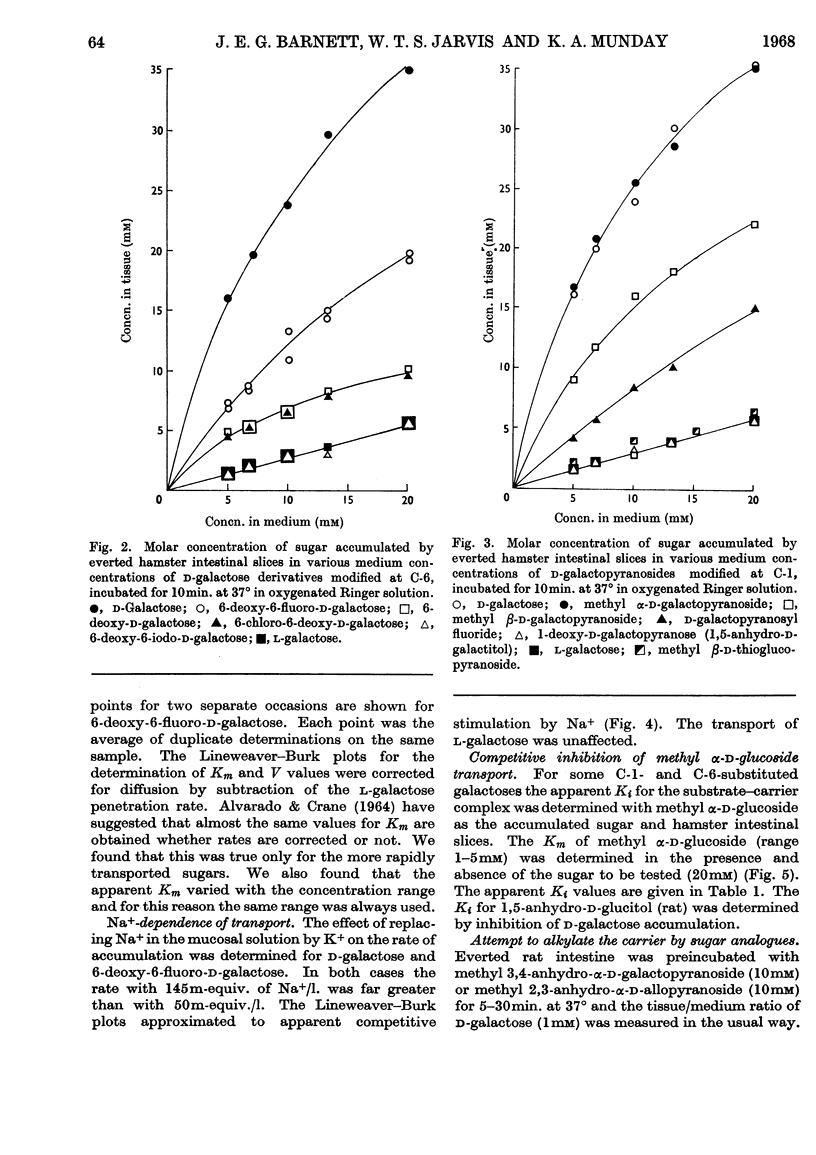
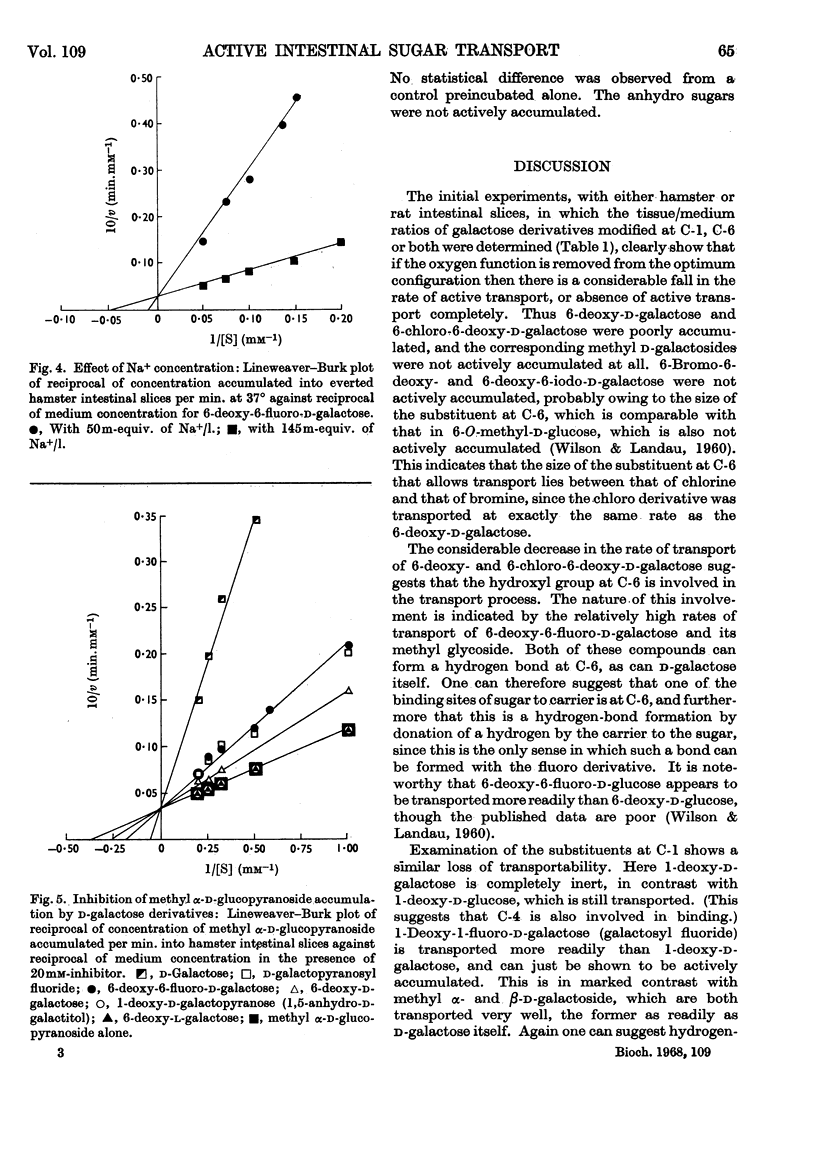
1. A series of d-galactose derivatives substituted at C-1 and C-6 were tested for active accumulation by everted segments of hamster and rat intestine. 2. d-Galactose and 6-deoxy-6-fluoro-d-galactose were accumulated far more rapidly than 6-deoxy- and 6-chloro-6-deoxy-d-galactose, and this is interpreted as due to hydrogen-bonding at C-6 during the transport process. 3. 6-Bromo-6-deoxy- and 6-deoxy-6-iodo-d-galactose were not actively transported, indicating that the allowed size of substituent at C-6 lies between that of chlorine and bromine atoms. 4. Similar results were obtained at C-1. Both methyl α-d-galactopyranoside and methyl β-d-galactopyranoside were well transported, but methyl β-d-thiogalactopyranoside and 1-deoxy-d-galactose were not transported; d-galactopyranosyl fluoride was transported, but only poorly. Again hydrogen-bonding is suggested. 5. It is proposed that d-glucose is the ideal structure for active transport and that binding occurs at C-1, C-2, C-3, C-4 and C-6. Loss of two or more of these bonds usually causes loss of active transport. 6. By plotting Lineweaver–Burk plots of the rates of transport of the galactose derivatives, the apparent V and Km values were obtained. With hamster intestine both these values were very reproducible. Contrary to expectation, V varied for different sugars. 7. The Ki of some of the analogues modified at C-1 and C-6 was determined with methyl α-d-glucoside as substrate. 8. An attempt to alkylate the carrier by using methyl 3,4-anhydro-α-d-galactoside was unsuccessful. There was no evidence that this compound was bound to the carrier.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F., CRANE R. K. STUDIES ON THE MECHANISM OF INTESTINAL ABSORPTION OF SUGARS. VII. PHENYLGLYCOSIDE TRANSPORT AND ITS POSSIBLE RELATIONSHIP TO PHLORIZIN INHIBITION OF THE ACTIVE TRANSPORT OF SUGARS BY THE SMALL INTESTINE. Biochim Biophys Acta. 1964 Oct 9;93:116–135. doi: 10.1016/0304-4165(64)90266-1. [DOI] [PubMed] [Google Scholar]
- Alvarado F. D-xylose active transport in the hamster small intestine. Biochim Biophys Acta. 1966 Feb 7;112(2):292–306. doi: 10.1016/0926-6585(66)90328-1. [DOI] [PubMed] [Google Scholar]
- BIHLER I., HAWKINS K. A., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. VI. The specificity and other properties of Na ion-dependent entrance of sugars into intestinal tissue under anaerobic conditions, in vitro. Biochim Biophys Acta. 1962 May 7;59:94–102. doi: 10.1016/0006-3002(62)90700-x. [DOI] [PubMed] [Google Scholar]
- Barnett J. E., Jarvis W. T., Munday K. A. Enzymic hydrolysis of the carbon-fluorine bond of alpha-D-glucosyl fluoride by rat intestinal mucosa. Localization of intestinal maltase. Biochem J. 1967 Jun;103(3):699–704. doi: 10.1042/bj1030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Jarvis W. T., Munday K. A. The hydrolysis of glycosyl fluorides by glycosidases. Biochem J. 1967 Nov;105(2):669–672. doi: 10.1042/bj1050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K. Intestinal absorption of sugars. Physiol Rev. 1960 Oct;40:789–825. doi: 10.1152/physrev.1960.40.4.789. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Csáky T. Z. Active transport of D-mannose in the small intestine. Life Sci. 1966 Jun;5(11):1025–1030. doi: 10.1016/0024-3205(66)90008-7. [DOI] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. Galactose absorption from the surviving small intestine of the rat. J Physiol. 1953 Feb 27;119(2-3):224–232. doi: 10.1113/jphysiol.1953.sp004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale R. J., Wiseman G. Active intestinal absorption of L-glucose. Nature. 1968 May 4;218(5140):473–474. doi: 10.1038/218473a0. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., CRANE R. K. The specificity of sugar transport by hamster intestine. Biochim Biophys Acta. 1958 Jul;29(1):30–32. doi: 10.1016/0006-3002(58)90142-2. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., LANDAU B. R. Specificity of sugar transport by the intestine of the hamster. Am J Physiol. 1960 Jan;198:99–102. doi: 10.1152/ajplegacy.1960.198.1.99. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., VINCENT T. N. Absorption of sugars in vitro by the intestine of the golden hamster. J Biol Chem. 1955 Oct;216(2):851–866. [PubMed] [Google Scholar]
- Wood K. R., Fisher D., Kent P. W. Fluorocarbohydrates. XIV. Reaction of N-(2-chloro-1,1,2-trifluoroethyl)diethylamine with some O-isopropylidene sugars. J Chem Soc Perkin 1. 1966;21:1994–1997. doi: 10.1039/j39660001994. [DOI] [PubMed] [Google Scholar]


