Abstract
1. Medium-chain fatty acyl-CoA synthetase (EC 6.2.1.2) was isolated by the method of Mahler, Wakil & Bock (1953) and the enzyme activity determined by the disappearance of CoA in the presence either of butyrate and ATP or of butyryl-AMP, as well as by ATP formation from butyryl-AMP and PPi. 2. Preincubation of the enzyme with CoA and ATP alone or together, followed by the removal of these substrates by gel filtration, caused a marked inhibition of ATP formation, contrary to results previously obtained with palmitoyl-CoA synthetase. 3. The effect of ATP on butyryl-AMP-dependent CoA disappearance was inconsistent. Low concentrations of ATP (0·1–0·5mm) always caused inhibition, whereas higher concentrations (5–10mm) activated in some enzyme preparations and inhibited in others. 4. This inconsistency was shown to be due to the presence of two enzyme fractions. Both fractions had similar activities when assayed by the butyryl-AMP- or butyrate-plus-ATP-dependent CoA disappearance. However, fraction I was activated by ATP as measured by butyryl-AMP-dependent CoA disappearance whereas fraction II was inhibited by it. Fraction I also catalysed ATP formation from butyryl-AMP and PPi whereas fraction II was lacking in such activity. 5. The relationship of these observations with respect to other known mechanisms of fatty acid-activating systems is discussed.
Full text
PDF


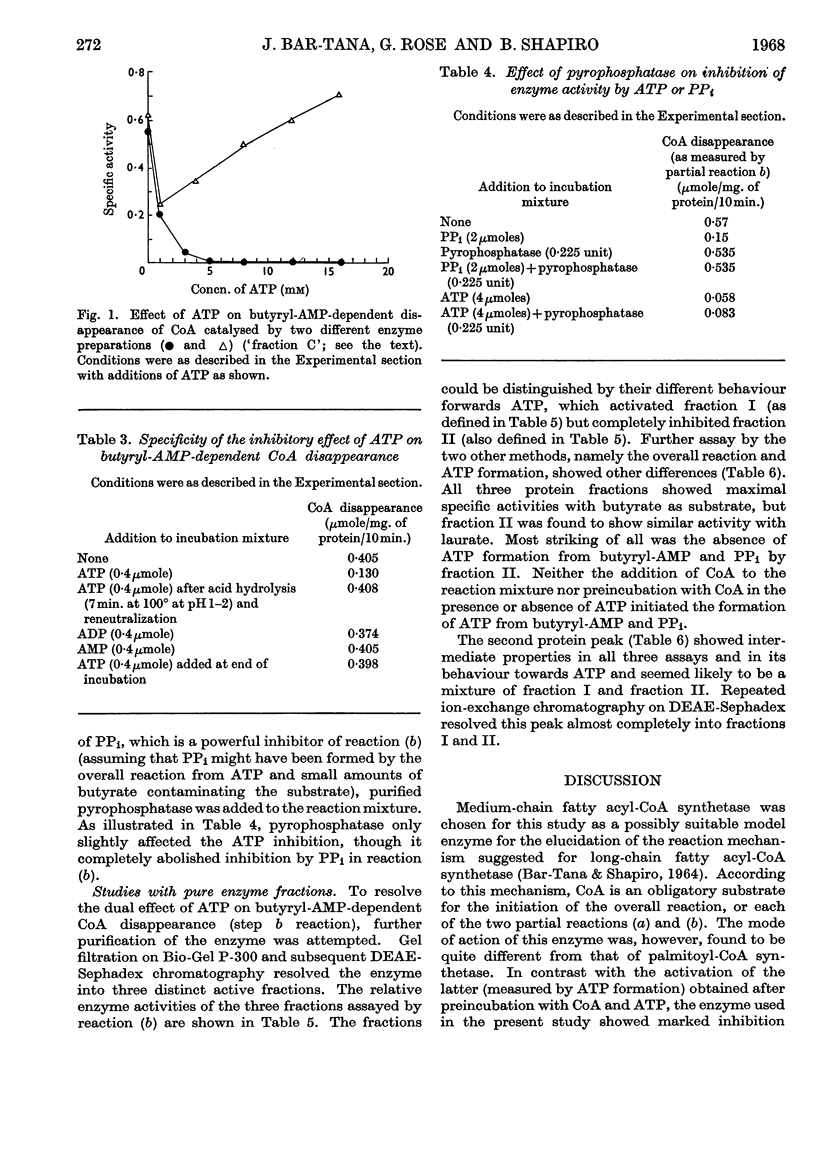
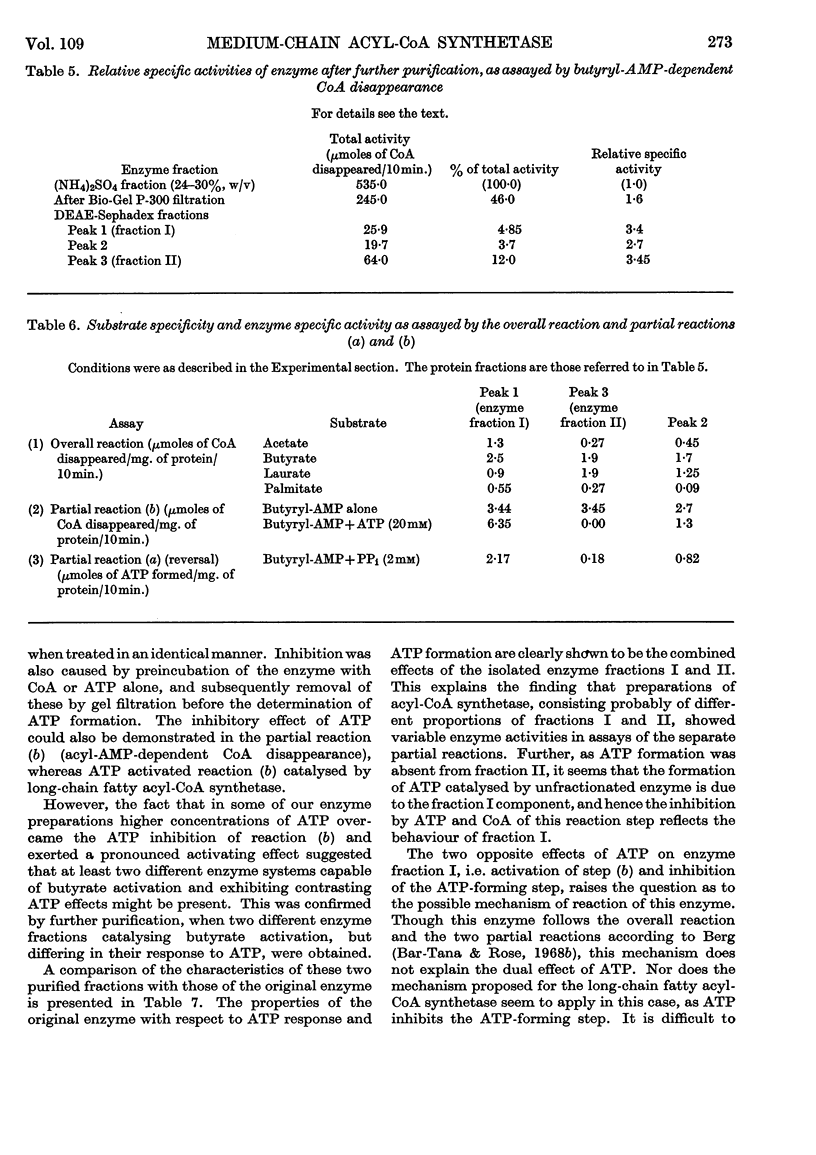

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG P. Acyl adenylates; an enzymatic mechanism of acetate activation. J Biol Chem. 1956 Oct;222(2):991–1013. [PubMed] [Google Scholar]
- Bar-Tana J., Rose G. Studies on medium-chain fatty acyl-coenzyme a synthetase. Enzyme fraction I: mechanism of reaction and allosteric properties. Biochem J. 1968 Sep;109(2):275–282. doi: 10.1042/bj1090275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J., Rose G. Studies on medium-chain fatty acyl-coenzyme a synthetase. Enzyme fraction II: mechanism of reaction and specific properties. Biochem J. 1968 Sep;109(2):283–292. doi: 10.1042/bj1090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J., Shapiro B. Studies on palmitoyl-coenzyme A synthetase. Biochem J. 1964 Dec;93(3):533–538. doi: 10.1042/bj0930533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S., Cha C. J., Parks R. E., Jr Succinic thiokinase. V. Preparation of labeled substrates, their bindings to the enzyme, and isotope exchange studies. J Biol Chem. 1967 Jun 10;242(11):2582–2592. [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAHLER H. R., WAKIL S. J., BOCK R. M. Studies on fatty acid oxidation. I. Enzymatic activation of fatty acids. J Biol Chem. 1953 Sep;204(1):453–468. [PubMed] [Google Scholar]
- Moyer R. H., Smith R. A. Succinic thiokinase: evidence of a bound 32P-3'-dephosphocoenzyme A. Biochem Biophys Res Commun. 1966 Mar 8;22(5):603–609. doi: 10.1016/0006-291x(66)90318-4. [DOI] [PubMed] [Google Scholar]
- Rao G. A., Johnston J. M. The involvement of bound-CoA in glyceride biosynthesis. Biochem Biophys Res Commun. 1966 Sep 8;24(5):696–700. doi: 10.1016/0006-291x(66)90380-9. [DOI] [PubMed] [Google Scholar]


