Abstract
1. A number of yeast species were examined for the presence of β-glucanases. Extracts obtained by cell disruption of Saccharomyces cerevisiae, Fabospora fragilis and Hansenula anomala hydrolysed laminarin and pustulan with the production of glucose. Enzymic activities were also detected in the culture fluids of F. fragilis and H. anomala grown aerobically in buffered mineral medium with glucose as the carbon source. 2. F. fragilis and H. anomala possessed approximately sevenfold higher β-(1→3)-glucanase activity than S. cerevisiae. 3. Intracellular exo-β-glucanase from baker's yeast was purified 344-fold from the dialysed cell extract. 4. Exo-β-glucanase from F. fragilis was purified 114-fold from the dialysed culture fluid and 423-fold from the dialysed intracellular extract. The purified extracellular and intracellular enzymes had similar properties and essentially the same specific activity, 79 enzyme units/mg. of protein. 5. Extracellular exo-β-glucanase of H. anomala was purified 600-fold. 6. The optimum pH of the enzymes from F. fragilis, S. cerevisiae and H. anomala was 5·5 in each case. Chromatographic evidence indicated that the three enzymes remove glucosyl units sequentially from laminarin as well as pustulan. 7. The ratio of activities towards laminarin and pustulan remained constant during purification of the exo-β-glucanase obtained from the three species, suggesting a single enzyme. Additional evidence for its unienzymic nature are: (i) the two activities were destroyed at exactly the same rate on heating of the purified enzyme from F. fragilis at three different temperatures; (ii) the competitive inhibitor glucono-δ-lactone gave the same value of Ki when tested with either substrate; (iii) quantitative application of the `mixed-substrate' method with the purified enzyme of S. cerevisiae gave data that were in excellent agreement with those calculated on the assumption of a single enzyme. 8. The purified exo-β-glucanases of the different species of yeast had different kinetic constants. The ratios of maximal velocities and Km values with laminarin and pustulan differed markedly. Comparison of Vmax. and Km values suggests that the rapid release of spores from asci in F. fragilis might be explained in terms of an enzyme with higher maximal velocity and higher affinity to the ascus wall than that present in baker's yeast. 9. The estimated molecular weights for exo-β-glucanases from F. fragilis, S. cerevisiae and H. anomala were 22000, 40000 and 30000 respectively.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON J. S. D., EDELMAN J. The carbohydrates of the Jerusalem artichoke and other Compositae. Biochem J. 1951 Jan;48(1):114–126. doi: 10.1042/bj0480114. [DOI] [PMC free article] [PubMed] [Google Scholar]
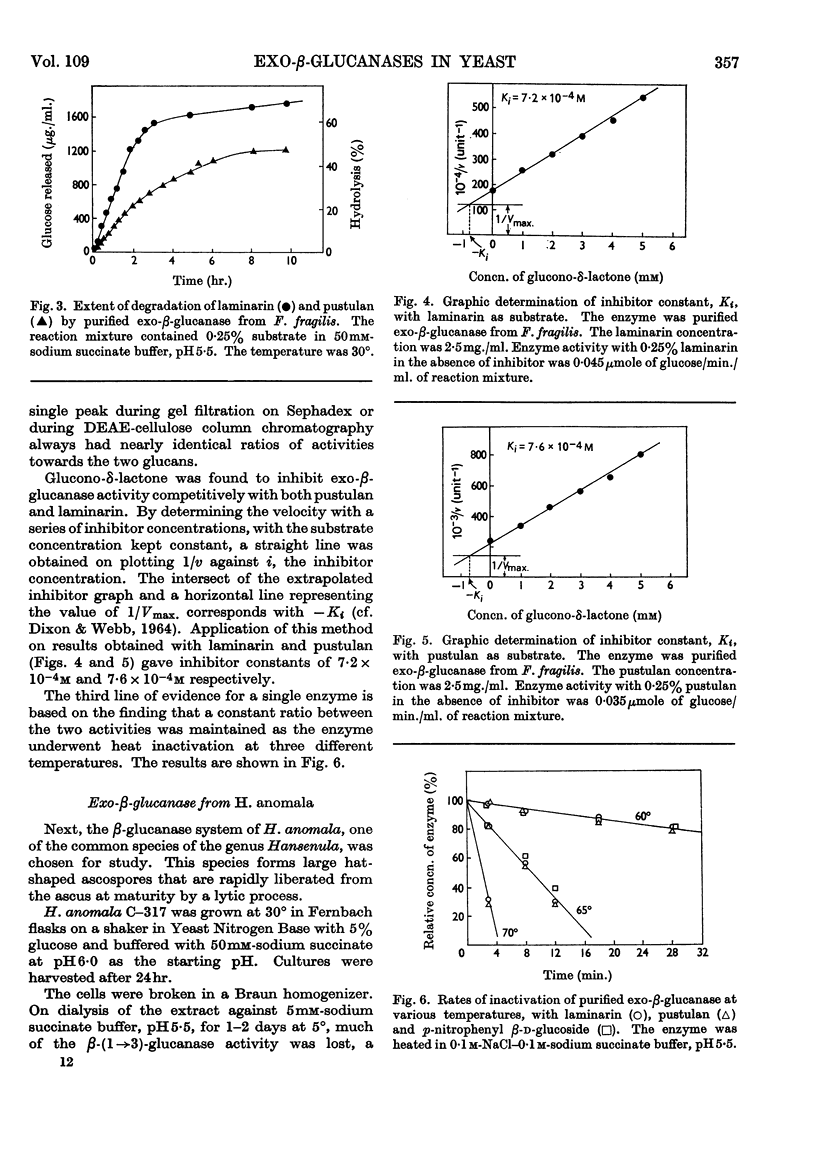
- BROCK T. D. Physiology of the conjugation process in the yeast Hansenula wingei. J Gen Microbiol. 1961 Nov;26:487–497. doi: 10.1099/00221287-26-3-487. [DOI] [PubMed] [Google Scholar]
- Bull A. T., Chesters C. G. The biochemistry of laminarin and the nature of laminarinase. Adv Enzymol Relat Areas Mol Biol. 1966;28:325–364. doi: 10.1002/9780470122730.ch5. [DOI] [PubMed] [Google Scholar]
- CONCHIE J., LEVVY G. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. Biochem J. 1957 Feb;65(2):389–395. doi: 10.1042/bj0650389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARVER J. C., EPSTEIN R. L. Method for rupturing large quantities of microorganisms. Appl Microbiol. 1959 Sep;7:318–319. doi: 10.1128/am.7.5.318-319.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- REESE E. T., PARRISH F. W., MANDELS M. Beta-d-1, 6-Glucanases in fungi. Can J Microbiol. 1962 Jun;8:327–334. doi: 10.1139/m62-045. [DOI] [PubMed] [Google Scholar]
- TANAKA H., PHAFF H. J. ENZYMATIC HYDROLYSIS OF YEAST CELL WALLS. I. ISOLATION OF WALL-DECOMPOSING ORGANISMS AND SEPARATION AND PURIFICATION OF LYTIC ENZYMES. J Bacteriol. 1965 Jun;89:1570–1580. doi: 10.1128/jb.89.6.1570-1580.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WEBB E. C., MORROW P. F. The activation of an arysulphatase from ox liver by chloride and other anions. Biochem J. 1959 Sep;73:7–15. doi: 10.1042/bj0730007. [DOI] [PMC free article] [PubMed] [Google Scholar]


