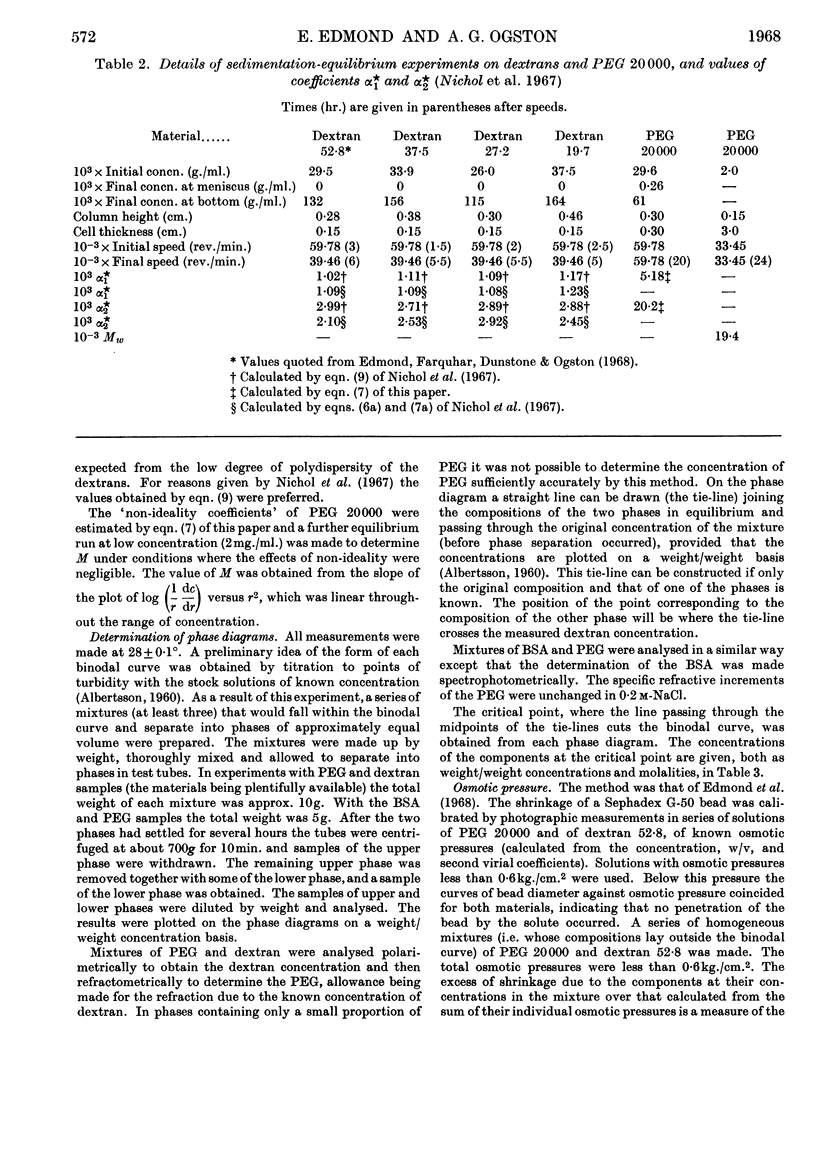
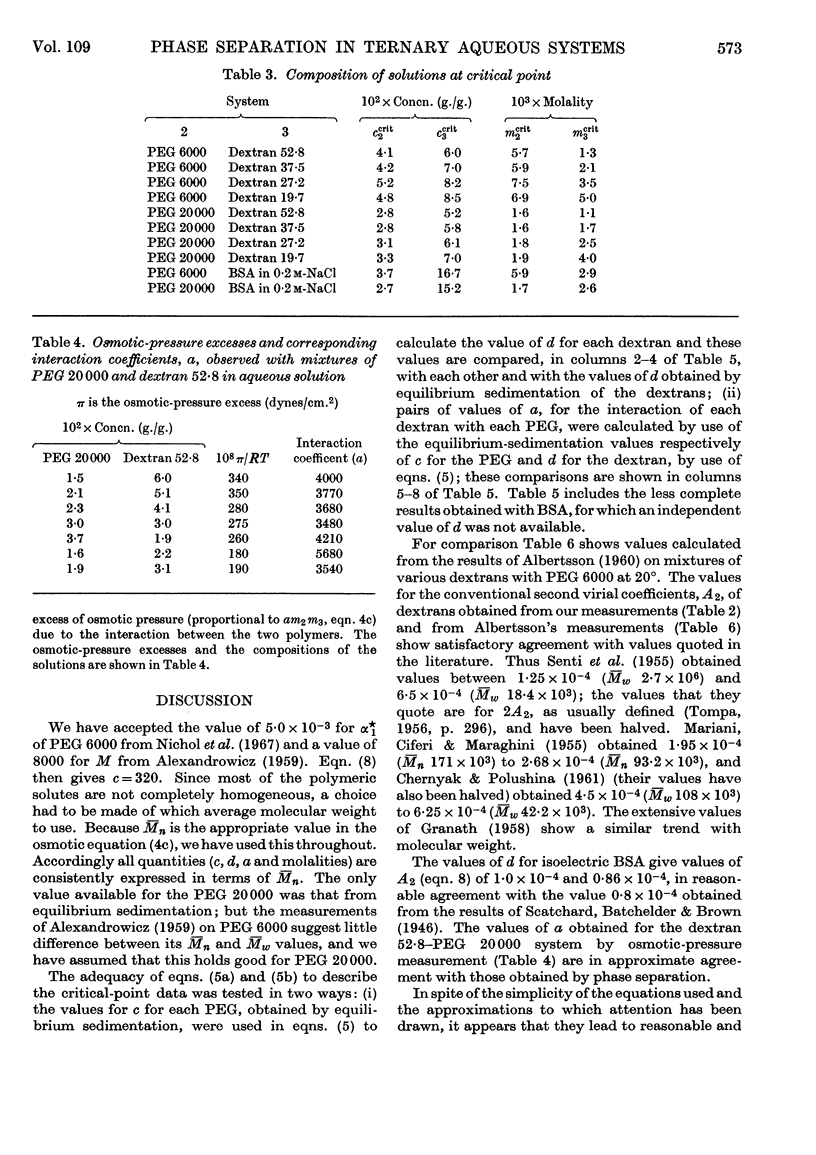
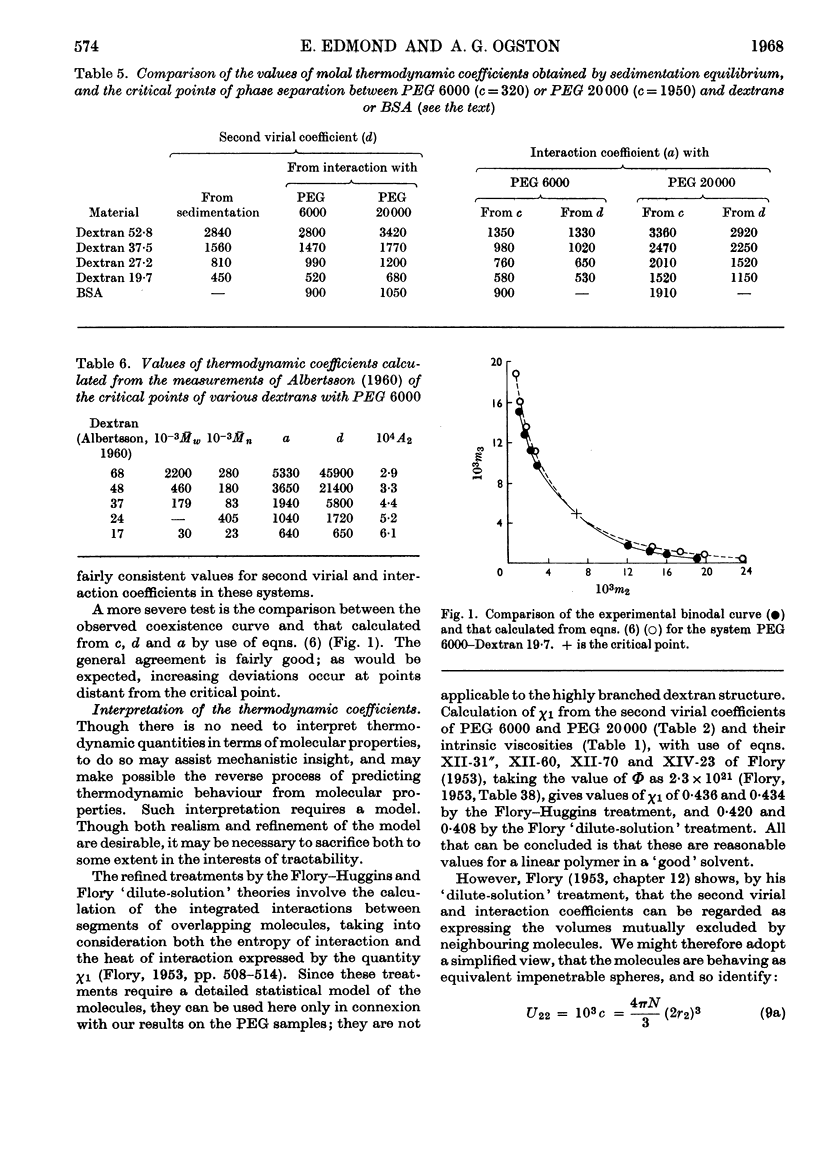
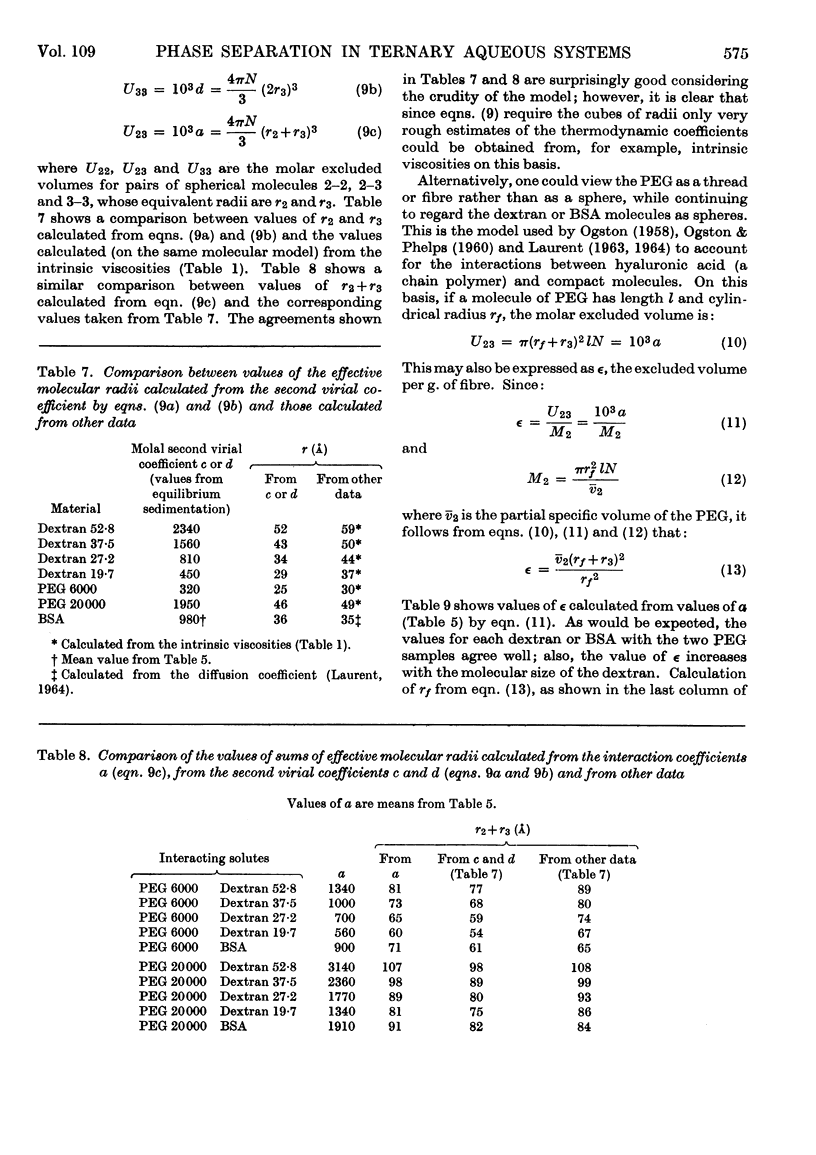
Abstract
1. Simple thermodynamic expressions are used to describe the properties of uncharged binary and ternary polymer solutions, in particular the sedimentation equilibrium of binary systems and the osmotic pressures and `incompatible' phase separations of ternary systems. 2. Sedimentation-equilibrium experiments were performed on four samples of dextran and two of polyethylene glycol. The critical points of the phase diagrams were determined for the mixed solutions of polyethylene glycol–dextran–water and of polyethylene glycol–bovine serum albumin–0·2m-sodium chloride solution. Osmotic pressures were measured on a single-phase mixed solution of a polyethylene glycol and a dextran. By use of the simple thermodynamic expressions consistent values of second virial and interaction coefficients for the materials used were obtained from these experiments. 3. The interpretation of the values of the second virial and interaction coefficients, on the basis of three models of molecular interaction, is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASASSA E. F., EISENBERG H. THERMODYNAMIC ANALYSIS OF MULTICOMPONENT SOLUTIONS. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- CHENIAK V. Ia, POLUSHINA T. V. [On the determination of the molecular weight of polyglucin]. Med Prom SSSR. 1961 Aug;8:39–43. [PubMed] [Google Scholar]
- Edmond E., Farquhar S., Dunstone J. R., Ogston A. G. The osmotic behaviour of Sephadex and its effects on chromatography. Biochem J. 1968 Aug;108(5):755–763. doi: 10.1042/bj1080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Laurent T. C. Precipitation of some plasma proteins by the addition of dextran or polyethylene glycol. Biochim Biophys Acta. 1967 Feb 21;133(2):371–373. doi: 10.1016/0005-2795(67)90079-7. [DOI] [PubMed] [Google Scholar]
- KRONMAN M. J., FOSTER J. F. Sedimentation and optical rotation behavior of bovine plasma albumin at low pH in the presence of various anions; effect of charge on molecular expansion. Arch Biochem Biophys. 1957 Nov;72(1):205–218. doi: 10.1016/0003-9861(57)90187-x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C. The interaction between polysaccharides and other macromolecules. 9. The exclusion of molecules from hyaluronic acid gels and solutions. Biochem J. 1964 Oct;93(1):106–112. doi: 10.1042/bj0930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol L. W., Ogston A. G., Preston B. N. The equilibrium sedimentation of hyaluronic acid and of two synthetic polymers. Biochem J. 1967 Feb;102(2):407–416. doi: 10.1042/bj1020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. The partition of solutes between buffer solutions and solutions containing hyaluronic acid. Biochem J. 1961 Apr;78:827–833. doi: 10.1042/bj0780827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G., Preston B. N. The exclusion of protein by hyaluronic acid. Measurement by light scattering. J Biol Chem. 1966 Jan 10;241(1):17–19. [PubMed] [Google Scholar]
- Ogston A. G. Some observations on mixtures of serum albumin and globulin. Biochem J. 1937 Nov;31(11):1952–1957. doi: 10.1042/bj0311952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. N., Davies M., Ogston A. G. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965 Aug;96(2):449–474. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]


