Abstract
Multiple system atrophy is an adult onset neurodegenerative disease, featuring parkinsonism, ataxia, and autonomic failure, in any combination. The condition is relentlessly progressive and responds poorly to treatment. Death occurs on average six to seven years after the onset of symptoms. No familial cases of multiple system atrophy have been reported, and no environmental factors have been robustly implicated as aetiological factors. However, analytical epidemiological studies are hampered because the condition is relatively rare. The discovery of the glial cytoplasmic inclusion (GCI) in 1989 helped to define multiple system atrophy as a clinicopathological entity, and drew attention to the prominent, if not primary, role played by the oligodendrocyte in the pathogenesis of the condition. Subsequently, GCIs were shown to be positive for α-synuclein, with immunostaining for this protein indicating that white matter pathology was more widespread than had previously been recognised. The presence of α-synuclein in GCIs provides a link with Parkinson's disease, dementia with Lewy bodies, and neurodegeneration with brain iron accumulation, type 1 (or Hallervorden-Spatz syndrome), in which α-synuclein is also found within Lewy bodies. This has led to the term “synucleinopathy” to embrace this group of conditions. The GCIs of multiple system atrophy contain a range of other cytoskeletal proteins. It is unknown how fibrillogenesis occurs, and whether there is primary oligodendrocytic dysfunction, which then disrupts the neurone/axon as a consequence of the glial pathology, or whether the oligodendrocytic changes merely represent an epiphenomenon. Further research into this devastating condition is urgently needed to improve our understanding of the pathogenesis, and also to produce new treatment approaches.
Keywords: multiple system atrophy, glial cytoplasmic inclusion, α-synuclein, oligodendrocyte
Multiple system atrophy (MSA) is a devastating adult onset, sporadic neurodegenerative disease of unknown aetiology, characterised by parkinsonism, ataxia, and autonomic failure, in any combination. The age adjusted prevalence of MSA in the UK is 4.4/100 000.1 The term “multiple system atrophy” was first proposed by Graham and Oppenheimer in 1969 to avoid “unnecessary confusion caused by inventing new names”.2 Recent consensus criteria recommend that patients with predominantly parkinsonian features should be designated MSA-P, whereas those with predominantly cerebellar features are designated MSA-C.3 These terms are more clinically appropriate than the pathologically derived terms striatonigral degeneration and olivopontocerebellar atrophy types of MSA, respectively.
The description of argyrophilic glial cytoplasmic inclusions (GCIs) in the oligodendrocytes of patients with phenotypically varied MSA provided persuasive evidence that MSA is a clinicopathological entity.4,5 Subsequently, similar inclusions were identified in glial nuclei (glial nuclear inclusions (GNIs)), and in neuronal cytoplasm and nuclei (neuronal cytoplasmic inclusions (NCIs), and neuronal nuclear inclusions (NNIs), respectively), and also in axons, although all at lower frequencies than the GCIs.6 However, the mechanisms underlying inclusion body formation, and how it relates to glial and neuronal loss in MSA, remain to be determined. Understanding these processes will be essential before an effective treatment to halt or reverse the disease can be developed.
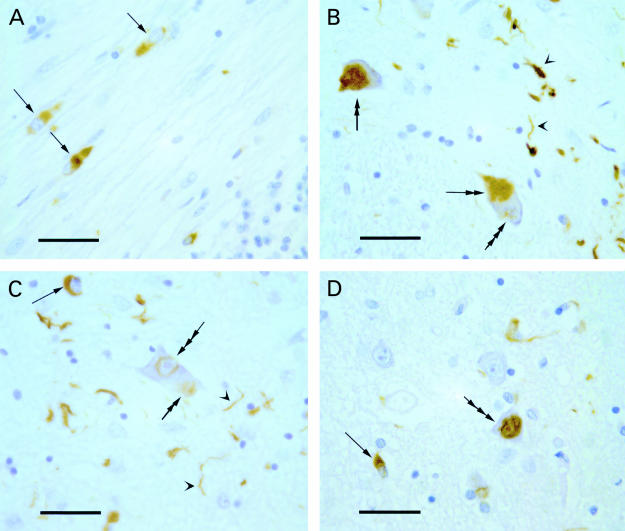
Recently, an abnormally insoluble filamentous/tubular form of α-synuclein protein was shown to be a major component of GCIs, NCIs, and NNIs, but not of GNIs (fig 1 ▶).7–10 The accumulation of this synaptic protein in MSA provides an unexpected and as yet unexplained link with Parkinson's disease, dementia with Lewy bodies, and neurodegeneration with brain iron accumulation, type 1 (NBIA 1 or Hallervorden-Spatz syndrome), in which α-synuclein is an important component of the Lewy bodies. MSA, Parkinson's disease, dementia with Lewy bodies, and NBIA 1 are thus sometimes referred to as “synucleinopathies”.11,12
Figure 1.
α-Synuclein pathology in multiple system atrophy. (A) Glial cytoplasmic inclusions (GCls) (arrows) in cerebellar white matter with cerebellar granule cells in the lower right corner. (B) Neuronal cytoplasmic inclusions (NCIs) (double arrows) and an early formation of a neuronal nuclear inclusion (NNI) (triple arrow) in neurones of pontine nuclei with neurites in the neuropil (arrowheads). (C) A GCI (arrow) and the early formation of an NCI (double arrow) and NNI (triple arrow) in a neurone of the pontine nuclei with neurites in the neuropil (arrowheads). (D) A GCI (arrow) and an NNI (triple arrow) in the pontine nuclei. Immunohistochemistry was performed using monoclonal antibodies to α-synuclein (Novocastra Laboratories, Newcastle upon Tyne, UK) on formalin fixed, paraffin wax embedded sections that had been pretreated with formic acid; Vectastain Elite ABC peroxidase kit (Vector, Peterborough, UK); DAB; Haematoxylin counterstain. Scale bars in A to D, 30 μm.
Presentation and natural history of MSA
Although parkinsonism develops in most patients with MSA (84–100%), “pure” cerebellar forms of the disorder are uncommon (0–16%).13 Autonomic failure was reported in 78% of 188 pathologically confirmed cases of MSA.14 Symptomatic orthostatic hypotension occurring within one year of onset of parkinsonism has recently been shown to predict MSA in 75% of cases, although this study was retrospective, and the numbers involved were small.15 The parkinsonism of MSA is more variably responsive to levodopa than Parkinson's disease itself. Up to one third of patients may show a moderate or good response to this drug, but this usually declines over the first one to two years of treatment.14
A male predominance of 1.4 : 1 was reported by Quinn in a review of 231 pathologically confirmed MSA cases.14 If confirmed, this observation may have aetiological relevance. For example, there may be greater environmental exposure to putative toxins in men, or endogenous protective factors (hormonal perhaps) in women.
The mean age at onset in 203 pathologically confirmed cases of MSA was 54.3 (range, 33–78) years.16 The upper limit of the age range must be viewed with a degree of caution, however, because clinicopathological series are prone to bias, and older patients are less likely to undergo postmortem examination.17 A population based study is necessary to confirm the age range and mean age of disease onset. The mean disease duration was only 6.2 (range, 0.5–24) years in a recent meta-analysis of 433 pathologically confirmed cases,18 indicative of a relentlessly progressive illness. This review was retrospective, however, and may have been biased towards the most severe cases. Cerebellar features were associated with marginally increased survival in this review, but this did not reach significance.
Neuropathology of MSA and clinicopathological correlation
Macroscopically, the brain in MSA shows varying degrees of atrophy of the cerebellum, cerebellar peduncles (especially the middle and inferior peduncles), pons, medulla, and also the posterolateral putamen. There may be loss of pigment in the substantia nigra and also discolouration of the striatum (notably the putamen). Excessive iron accumulation has been demonstrated within the striatum to account for this pigmentary change.19
Oligodendrocyte GCIs and GNIs have a so called “system bound” distribution in the suprasegmental motor systems (primary motor, and higher motor areas of the cerebral cortex, pyramidal, extrapyramidal, and corticocerebellar systems), in the supraspinal autonomic systems, and in their targets.20–22 Neuropathological changes in neurones follow a similar system bound distribution and include variable neuronal loss, and densities of NCIs and NNIs in the striatum, substantia nigra, locus ceruleus, inferior olives, pontine nuclei, cerebellar Purkinje cells, dorsal motor nucleus of vagus, nucleus vestibularis, intermediolateral cell column of the spinal cord, and Onuf's nucleus.16,23 Rare MSA cases showing additional involvement of frontal or temporal lobes, including atrophy, and the presence of GCIs and NCIs have also been reported.24,25 White matter pathology is also increasingly recognised in MSA, with the fibre tracts of the suprasegmental motor and supraspinal autonomic systems (see above) bearing the brunt of demyelination.26 Furthermore, using monoclonal and polyclonal antibodies that recognise epitope QDENPVV of human myelin basic protein, accesible only in areas of myelin degeneraiton, Matsue and colleagues have demonstrated that in MSA myelin degeneration and abnormal oligodendrocytes were widespread, even in areas where GCIs were not detectable, and where myelin appeared intact with standard myelin stains.27
A correlation has been established between akinesia and the degree of nigral and putaminal cell loss, although rigidity relates only to this last feature.16 Ataxia correlates with the degree of olivopontocerebellar atrophy and pyramidal signs with pyramidal tract pallor.16 Recently, a loss of Betz cells was documented in all of seven patients with MSA studied, six of whom had pyramidal signs documented before death.28 Some groups have found an association between postural hypotension and intermediolateral cell column degeneration,16 but this finding has not been confirmed by others.29 A severe loss of catecholaminergic neurones in the rostral ventrolateral medulla has been noted in patients with MSA.30 This area is involved in the control of sympathetic cardiovascular outflow.
There is limited information available about striatal dopamine receptors in MSA and their correlation with extrapyramidal features. A relative preservation of putaminal cell counts in patients with MSA responding to levodopa has been reported,31 and resistance to levodopa might be the result of a loss of putaminal dopamine D2 receptors.32 Patients with MSA who are not responsive to levodopa may have more severe topographical degeneration of the putaminal efferent terminals in the ventrolateral portion of the globus pallidus.33 In contrast, a case of MSA has been reported where there was no significant response to dopaminergic treatment, yet there was no evidence of putaminal cell loss at necropsy.34 Furthermore, in vivo positron emission tomography (PET) studies using the dopamine D2 receptor ligand 11C-raclopride have demonstrated only a modest 15% reduction in striatal D2 sites.35 The failure to respond to levodopa was thought to reflect “loss of other basal ganglia connections”.
Genes, polymorphisms, and MSA
MSA, as reflected in its current definition, is regarded as a sporadic disease.36 Familial cases have not been described, although clinical symptoms of MSA were reported by a significantly larger group of patients' relatives than controls in one study.37 However, a self administered questionnaire was used to elicit symptoms from the relatives in this series, leading to potential bias.
Several studies have looked for polymorphisms or mutations in candidate genes, which may predispose an individual towards developing MSA. The apolipoprotein ɛ4 allele is not over-represented in MSA when compared with controls, and there have been conflicting reports of the association of a cytochrome P-450-2D6 polymorphism with MSA.38–40 There is no evidence to suggest that patients with MSA have expansions at the SCA1 and SCA3 alleles.41 Furthermore, there is no evidence to support an association between polymorphisms in the H5 pore region of the human homologue of the weaver mouse gene, hiGIRK2, the insulin-like growth factor 1 receptor gene (linked with a decreased intracellular response to insulin-like growth factor 1 in the cerebellar cortex of lurcher mice), or the ciliary neurotrophic factor gene.41
It seems improbable that a mutation in the α-synuclein gene underlies protein accumulation in MSA. Recent studies have not found a mutation in the entire coding region of the α-synuclein gene in patients with pathologically confirmed MSA.42,43 However, mutations in the regulatory or intronic regions of the gene have not been ruled out.
Polymorphisms in the α-synuclein gene might increase the risk of developing MSA, by promoting α-synuclein protein aggregation. To date, polymorphisms have been identified in the promoter sequence, and in the intron 4 sequence of the α-synuclein gene.44 A combination of allele 1 of the α-synuclein promoter polymorphism and the ApoE4 allele has been reported to increase the relative risk for developing sporadic Parkinson's disease 12.8 fold.45 Polymorphisms in codons 1 to 39 of the α-synuclein gene, a domain related to interaction with the recently identified protein, synphilin-1, or polymorphisms in the synphilin-1 gene itself, or in the genes of other protein interacting partners of α-synuclein, may also need to be considered.46 The number of α-synuclein protein interacting partners has expanded to include 14-3-3 protein chaperones, protein kinase C, extracellular regulated kinase, and BAD, a Bcl-2 homologue that regulates cell death.47
Increased expression of a brain specific protein called ZNF231 in cerebellar neurones has been reported to occur in patients with MSA.48 The gene is located on chromosome 3p21 and encodes a neuronal double zinc finger protein with a nuclear targeting sequence, suggesting that it might function as a transcription regulator. The importance of this finding is as yet uncertain, but it is possible that patients with MSA differ from unaffected individuals by sequence polymorphisms within, and flanking, the putative functional motifs of the ZNF231 gene.
Glial cytoplasmic inclusions: characteristics and composition
Argyrophilic oligodendroglial inclusions were first described in the brains of patients with MSA in 1989 and became known as glial cytoplasmic inclusions.4 Subsequent studies have confirmed the sensitivity and specificity of GCIs for MSA.5,21,49 Double staining techniques using markers for oligodendroglia, such as myelin basic protein, Leu-7, carbonic anhydrase enzyme II, and transferrin, confirm the localisation of the inclusion to this cell type.49,50 In contrast, GCI containing cells stain negatively for glial fibrillary acidic protein (an astrocytic marker), and for class II major histocompatibility antigen (a microglial marker).
Using routine light microscopy, GCIs are faint eosinophilic inclusions that eccentrically displace the nucleus. By virtue of its selective dark staining of inclusions with a clean background, the Gallyas silver technique is the method of choice for demonstrating GCIs. This technique shows that the GCIs vary in morphology from sickle shaped to flame shaped to ovoid, and occasionally superficially resemble neurofibrillary tangles.51 GCIs are essentially negative with other commonly used stains, including phosphotungstic acid haematoxylin, periodic acid Schiff, Masson trichrome, Alcian blue, thioflavine S, Congo red, oil red O, and Sudan black B.23,52
At the ultrastructural level, GCIs are non-membrane bound cytoplasmic inclusions composed of loosely aggregated filaments/tubular structures (20–40 nm in cross sectional diameter) and granular material that may ensnare cytoplasmic organelles, such as mitochondria and secretory vesicles.4–6,49
Table 1 ▶ lists the immunocytochemical characteristics of GCIs. Notably, GCIs are immunoreactive for ubiquitin and are generally negative for tau. Conflicting data exist regarding whether GCIs stain for tau, but it is now believed that if the inclusions contain tau, it is largely non-phosphorylated, in contrast with the neurofibrillary pathology of Alzheimer's disease.53 Thus, phosphate dependent tau antibodies will not label GCIs in MSA. GCIs are immunoreactive with several other cytoskeletal proteins, including α-tubulin and β-tubulin,4 mitogen activated protein 5 (MAP5),54 MAP2,55 and also cyclin dependent kinase 5 (cdk5) and MAP kinase (MAPK), both of which are known to phosphorylate MAP2.55 This led to the hypothesis that there is a close association between the GCI and the microtubular cytoskeleton, with aberrant or ectopic expression of the neuronal kinases cdk5 and MAPK causing abnormal phosphorylation of microtubular cytoskeletal proteins.55
Table 1.
| Positive immunoreactivity | Negative immunoreactivity |
| α-Synuclein | Tau* |
| Ubiquitin | Neurofilaments |
| αB-crystallin | Glial fibrillary acidic protein |
| α-Tubulin and β-tubulin | Myelin basic protein |
| Mitogen activated protein 2 | Vimentin |
| Mitogen activated protein 5 | Actin |
| Cyclin dependent kinase 5 | Desmin |
| Mitogen activated protein kinase | Myosin |
| Midkine | Cytokeratin |
| Rab5 | |
| Rabaptin-5 |
*Glial cytoplasmic inclusions are generally negative for phosphate dependent tau antibodies and positive for normal adult tau.
The small heat shock protein and molecular chaperone, αB-crystallin, is a normal component of the central nervous system, where it is expressed primarily in oligodendrocytes and to a lesser degree in astrocytes.57 It is also an important protein component of GCIs. αB-crystallin binds to 20S proteasome, thereby regulating its proteolytic activity,56 in addition to binding to intermediate filaments.57
Ubiquitin is also involved in the 26S proteasome dependent proteolytic process and may play a protective role against neurodegeneration.58 Although the inclusions of MSA may be identified by ubiquitin immunostaining, results on isolated GCI proteins suggest that they are poorly ubiquitinated.56
In sections of MSA brains, antibodies to α-synuclein immunolabel a greater number of GCIs than do anti-ubiquitin antibodies, indicating that α-synuclein is a major component of GCIs, and that the accumulation of α-synuclein precedes its ubiquitination.8,11 α-Synuclein, also referred to as the precursor of the non-amyloid component of plaques (NACP), is a 140 amino acid protein that is normally localised in the human brain to presynaptic nerve terminals.59 It is natively unfolded and highly soluble,60 but can polymerise into filaments under a variety of in vitro conditions, including increased temperature and concentration, acidic pH conditions, longer time lag, and increased iron concentrations.61–64 Hence, the formation and accumulation of α-synuclein filaments in GCIs, and in Lewy bodies,7–9,11 has been speculated to result from altered intracellular conditions.61,62 It is of interest that in the basal ganglia of patients with MSA, total iron concentrations are raised,65 and GCIs have been found within oligodendroglial cells containing iron pigment, although inclusions have also been found in cells with no evidence of pigment accumulation.19 Oligodendrocytes are the predominant iron regulatory cells in the brain,66 but it is not known whether oligodendrocytes in patients with MSA show abnormal concentrations or activities of ferritin (the iron sequestration protein) or transferrin (the iron transport protein).
Full length α-synuclein is present in GCIs and NCIs,11,67 although more vigorous antigen retrieval is required for immunohistochemical detection of other than C-terminal epitopes.67 In contrast, very little full length α-synuclein appears to be present in immunoisolated GCIs, and the C-terminal truncated form of α-synuclein may predominate.56 In addition to the formation of GCIs, there is evidence for a more widespread modification of α-synuclein solubility in MSA than is obvious from the GCI distribution.26 In particular, in MSA, and also in preliminary studies with Lewy body disease, an increased ratio of sodium docecyl sulphate soluble to buffer soluble α-synuclein has been seen, leading to the proposal that this property may be a biochemical “fingerprint” for the synucleinopathies, even in the absence of inclusion bodies.26
Using immunoelectron microscopy on isolated sarcosyl insoluble filaments extracted from MSA brains, and PER4 antiserum to the C-terminus of α-synuclein, some filaments appear to be twisted, with a width alternating between 5 and 18 nm, and an apparent period of 70–90 nm, whereas other filaments appear to be straight, with a uniform width of 10 nm.11 The differences in morphology and diameter between the isolated α-synuclein filaments and the aggregated filamentous/tubular structures seen in sections of GCIs (see above) are thought to indicate that although α-synuclein may play a key role in fibrillogenesis, other cytoskeletal proteins (for instance α-tubulin and β-tubulin) are involved in filament formation.56,62 Furthermore, non-cytoskeletal elements also appear to be involved, as demonstrated most recently with antibodies to midkine, a new neurotrophic factor found to label most GCIs intensely. With immunoelectron microscopy, midkine positive, granule coated fibrils appear to be essential constituents of GCIs.68 Midkine is a heparin binding growth factor, implicated in various biological phenomena such as neuronal survival, differentiation, and migration, angiogenesis, and carcinogenesis.69 Midkine is strongly expressed in the nervous system during the midgestation period, probably by astrocytes. It is absent from normal adult brain, except in ischaemic zones surrounding cerebral infarction.70
The reason for the expression and accumulation of α-synuclein, a nerve terminal protein, in the GCIs of MSA brains is unknown. In cultured rat oligodendrocytes the expression of α-synuclein mRNA and protein is developmentally regulated,71 hence the accumulation is more likely to be a consequence of altered rather than de novo expression. One possibility is that oligodendrocytes in MSA brains may have an impaired ability to degrade α-synuclein, which they normally produce at very low concentrations.9 Alternatively, selective upregulation in the expression of α-synuclein in glial cells could occur in response to certain pathologies.7 The accumulation in GCIs of tau and MAP2 also appears to be a consequence of altered rather than de novo synthesis because both of these principally neuronal proteins are expressed in immature oligodendrocytes grown without axonal contact in tissue culture.72,73 The aberrant expression of the neuronal kinases cdk5 and MAPK (see above), of the neuronal endocytosis regulatory proteins Rab5 and Rabaptin-5,74 and of the neuronal survival and differentiation factor midkine (see above), normally expressed by astrocytes, suggests even more profound alterations in the phenotype of the MSA oligodendrocytes. Whether this is indicative of the existence of a repair mechanism68 or of a more profound defect in the oligodendrocyte–axon–neurone communication, involving oligodendroglial/neuronal trophic factors, remains to be established.
The neuronal inclusions (NCI and NNI) in MSA are less frequent than GCIs, and their ultrastructure reveals a composition of granules and filaments that tend to be associated with a more diverse range of cellular organelles, when compared with GCIs. In common with GCIs, however, neuronal inclusions stain positively both for ubiquitin and α-synuclein.19 Antibodies to α-synuclein, but not to ubiquitin, also reveal numerous degenerating neurites in the white matter of patients with MSA.8,26 This suggests that a hitherto unrecognised degree of pathology may be present in the axons of patients with MSA, although whether neuronal/axonal α-synuclein pathology precedes glial α-synuclein pathology or myelin degeneration (see above) has not yet been determined.
Cell death of oligodendrocytes and neurones in MSA
It is something of a paradox that although MSA produces clinical symptoms typical of grey matter dysfunction, the hallmark pathological lesion affects myelin producing cells.51 How oligodendroglial dysfunction might lead to regional neuronal loss remains unexplained. There is no evidence to suggest that oligodendrocytes can be subtyped according to the neuronal populations they subserve. Indeed, single oligodendrocytes may myelinate axons of different anatomical tracts. Furthermore, GCIs involve all morphological types of oligodendrocytes (perivascular, perifascicular, and perineuronal) with varying frequency in different anatomical regions.23 There are therefore no clues to point towards a “selective vulnerability” of a particular subgroup of oligodendrocytes. Nevertheless, because GCIs and oligodendroglial loss show a pronounced preponderance over NCIs and neuronal loss, it has been suggested that oligodendroglial pathology may be the primary lesion of MSA, rather than an epiphenomenon.21–23 It has also been shown that in MSA pronounced DNA fragmentation, characteristic of apoptotic cell death, occurs almost exclusively in oligodendrocytes in a distribution pattern similar to that of GCIs.22 However, it appears that GCI formation may represent a different stage in oligodendroglial pathology because only oligodendrocytes lacking GCIs express Bax, the apoptosis promoting protein. In contrast, the GCI containing oligodendrocytes express Bcl-2, the apoptosis suppressor protein, indicative of an activated repair mechanism.22 This raises the possibility that in MSA regional neuronal/axonal pathology and withdrawal of axon derived trophic factors normally preventing apoptosis (see below) precede the dysfunction of oligodendrocytes, and demyelination in the related white fibre tracts.
The absence of DNA fragmentation in MSA neurones may indicate that they are destroyed either by necrosis or by a form of programmed cell death other than apoptosis.22 Nonetheless, increased p53 immunoreactivity in the striatal and midbrain neurones of patients with MSA has been interpreted as evidence of neuronal apoptosis75 because p53 gene upregulation is known to precede apoptosis. More recently, immunoreactivity for the calcium binding proteins calbindin and parvalbumin has been shown to be greatly decreased in the cerebellar Purkinje cells of patients with MSA, whereas immunoreactivity for both Bax, the apoptosis promoting protein, and the Bcl-x, the apoptosis suppressor protein, was increased. The expression of Bcl-2, another apoptosis suppressor protein, was restricted to a subpopulation of granule neurones.76 It was suggested that a diminished calcium binding capacity of MSA Purkinje cells could lead to a change in the regulation of proteins of the Bcl-2 family, favouring the initiation of apoptosis.
A further clue to the regional selectivity seen in MSA and apoptosis may lie in the study of a family of cysteine proteases called caspases, which act as the executioners of apoptosis. Cytoskeletal proteins and enzymes essential for cell repair may be substrates for caspase processing in several neurodegenerative diseases.77 Caspase-3 cleaves the antiapoptotic protein Bcl-2. Peptide caspase inhibitors have been shown to protect against 1-methyl-4-phenylpyridinium induced apoptosis in cultured cerebellar granule neurones.78 Furthermore, the distribution of caspase-3 may contribute towards the regional vulnerability seen in the substantia nigra in idiopathic Parkinson's disease, with dopaminergic neurones expressing caspase-3 degenerating preferentially.79 Similar studies in MSA have not yet been reported.
The activation of microglial cells may be the final common pathway, contributing both to demyelination and neuronal removal, irrespective of the mode of cell death. Microglia express proinflammatory peptides, which may be a result of activation of nuclear factor κB (NF-κB). Affected brain areas of patients with MSA show strong immunoreactivity for nuclear Rel A p65 (a subunit of the NF-κB/Rel family), which is almost exclusively localised in activated microglia. Nuclear translocation of Rel A is not detected in striatal tissue of normal controls and patients with Parkinson's disease. This suggests that NF-κB/Rel A complexes may play a role in mediating microglial activation in MSA.80 Finally, PK 11195 selectively binds to benzodiazepine sites on activated microglia. 11C PK 11195 positron emission tomography has recently demonstrated activated microglia in vivo in the putamen, pallidum, substantia nigra, and pontine region in four patients with MSA.81
Trophic factors and extracellular matrix
Trophic factors have been studied in several neurodegenerative diseases, both for their potential pathogenic role and also for their possible therapeutic benefit. Brain derived neurotrophic factor (BDNF) is abundantly and widely expressed in neurones of the adult mammalian brain. It has a neurotrophic effect on many neuronal types, including nigral dopaminergic and striatal neurones.82 BDNF positive neurites have been shown to be more abundant in the striatum of patients with MSA than in the striatum of those with Parkinson's disease and normal controls. The upregulation of BDNF has been interpreted as a protective mechanism against progressive degeneration of the striatal neurones in MSA.83 However, it is not clear whether the upregulation has occurred in the major striatal afferents originating in the cortex or in the minor afferents originating in the substantia nigra. The striatal neurones themselves are not BDNF immunoreactive.82 So far, there have been no reports of alterations in glial growth factors in patients with MSA. Glial growth factors are derived from both astrocytes and neurones. White matter astrocytes produce platelet derived growth factor (PDGF), which has been shown to act as a survival factor for newly formed oligodendrocytes.84 Neurones/axons generate several isoforms of neuregulins, which are members of a large trophic family all originating from a single gene. Some of the isoforms promote proliferation and survival of oligodendrocytes through their interaction with the erb-B family of receptors, expressed by the oligodendrocytes.85 Glial growth factor, one of the neuregulin isoforms, promotes the proliferation and survival of oligodendrocyte progenitors but inhibits their further differentiation.86 ARIA, another neuregulin isoform, acts as a morphogen for developing oligodendrocytes by promoting the extension and complexity of their processes.87
Most recently, it has been demonstrated that the sensitivity of oligodendrocytes to astrocyte derived PDGF is greatly enhanced by an extracellular matrix glycoprotein, laminin-2,88 which is expressed by Purkinje cell axons.89 Laminin-2 also appears to play an important part in the signalling mechanisms that stimulate oligodendrocytes to elaborate the myelin sheath.90 Laminin-2 is a heterotrimer composed of a longer α2 chain and shorter β1 and γ1 chains. The α2 chain is associated with neuritic processes, synapses, and dendritic spines of limbic neuronal populations, whereas the γ1 chain is found in cell bodies of essentially all neurones in the central nervous system.91,92 Based on the restricted distribution of the α2 chain to the limbic areas, it has been proposed that isoforms of the laminin α chains, other than the already known α1 and α2 chains, are expressed by different neuronal systems.91 Thus, it is conceivable that polymorphisms in the genes encoding laminin α chains, which are shared by the suprasegmental motor system, the supraspinal autonomic systems, and their targets, could be risk factors for MSA, and may underlie the system bound distribution of the oligodendroglial and neuronal pathology in this disease.
Conclusion
The aetiology of MSA remains elusive. The discovery of α-synuclein within GCIs has provided a recent impetus to research, and also an elusive link with Parkinson's disease. Nevertheless, MSA is distinguished from other neurodegenerative diseases by the prominent, if not primary, involvement of the glial cell. In contrast with Parkinson's disease, dementia with Lewy bodies, and a host of other neurodegenerative tauopathies, familial MSA has never been documented, and there are no clues as yet to a genetic predisposition.
It is likely that future progress in unravelling the cause of MSA will need to include large multicentre clinical studies, applying validated diagnostic criteria to yield a sizeable cohort of well characterised MSA cases. This cohort will form the necessary basis for genetic and pathological studies. The potentially central role of α-synuclein in fibrillogenesis, and its interaction with other cytoskeletal proteins within glial cells is likely to provide a fruitful avenue for further research. At the same time, the temporal and spatial involvement of the pathological process in MSA needs to be investigated in more detail, and potentially disruptive factors of the oligodendrocyte–neurone–axon functional unit should be explored further.
References
- 1.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 1999;354:1771–5. [DOI] [PubMed] [Google Scholar]
- 2.Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 1969;32:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–8. [DOI] [PubMed] [Google Scholar]
- 4.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy Drager syndrome). J Neurol Sci 1989;94:79–100. [DOI] [PubMed] [Google Scholar]
- 5.Kato S, Nakamura H, Hirano A, et al. Argyrophilic ubiquitinated cytoplasmic inclusions of Leu-7-positive glial cells in olivopontocerebellar atrophy (multiple system atrophy). Acta Neuropathol 1991;82:488–93. [DOI] [PubMed] [Google Scholar]
- 6.Papp MI, Lantos PL. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci 1992;107:172–82. [DOI] [PubMed] [Google Scholar]
- 7.Arima K, Ueda K, Sunohara N, et al. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol (Berl) 1998;96:439–44. [DOI] [PubMed] [Google Scholar]
- 8.Gai WP, Power JH, Blumbergs PC, et al. Multiple-system atrophy: a new alpha-synuclein disease? Lancet 1998;352:547–8. [DOI] [PubMed] [Google Scholar]
- 9.Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol 1998;44:415–22. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi K, Hayashi S, Kakita A, et al. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol (Berl) 1998;96:445–52. [DOI] [PubMed] [Google Scholar]
- 11.Spillantini MG, Crowther RA, Jakes R, et al. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 1998;251:205–8. [DOI] [PubMed] [Google Scholar]
- 12.Galvin JE, Giasson B, Hurtig HI, et al. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. Am J Pathol 2000;157:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenning GK, Quinn NP. Parkinsonism. Multiple system atrophy. Baillieres Clin Neurol 1997;6:187–204. [PubMed] [Google Scholar]
- 14.Quinn N. Multiple system atrophy. In: Marsden CD, Fahn S, eds. Movement disorders 3. Oxford: Butterworth-Heinemann; 1994:262–81.
- 15.Wenning GK, Scherfler C, Granata R, et al. Time course of symptomatic orthostatic hypotension and urinary incontinence in patients with postmortem confirmed parkinsonian syndromes: a clinicopathological study. J Neurol Neurosurg Psychiatry 1999;67:620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenning GK, Tison F, Ben Shlomo Y, et al. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 1997;12:133–47. [DOI] [PubMed] [Google Scholar]
- 17.Maraganore DM, Anderson DW, Bower JH, et al. Potential selection bias in autopsy series of Parkinson's disease and related disorders. Neurology 1998;50(suppl 4):A98–9. [Google Scholar]
- 18.Ben-Shlomo Y, Wenning GK, Tison F, et al. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology 1997;48:384–93. [DOI] [PubMed] [Google Scholar]
- 19.Dickson DW, Lin W, Liu WK, et al. Multiple system atrophy: a sporadic synucleinopathy. Brain Pathol 1999;9:721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 1994;117:235–43. [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Yagishita S, Ryo M, et al. The distribution and dynamic density of oligodendroglial cytoplasmic inclusions (GCIs) in multiple system atrophy: a correlation between the density of GCIs and the degree of involvement of striatonigral and olivopontocerebellar systems. Acta Neuropathol (Berl) 1997;93:585–91. [DOI] [PubMed] [Google Scholar]
- 22.Probst-Cousin S, Rickert CH, Schmid KW, et al. Cell death mechanisms in multiple system atrophy. J Neuropathol Exp Neurol 1998;57:814–21. [DOI] [PubMed] [Google Scholar]
- 23.Lantos PL, Papp MI. Cellular pathology of multiple system atrophy: a review. J Neurol Neurosurg Psychiatry 1994;57:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konagaya M, Sakai M, Matsuoka Y, et al. Multiple system atrophy with remarkable frontal lobe atrophy. Acta Neuropathol (Berl) 1999;97:423–8. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya K, Nagatomo H, Iwabuchi K, et al. Asymmetrical temporal lobe atrophy with massive neuronal inclusions in multiple system atrophy. J Neurol Sci 2000;179:50–8. [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW, Liu W, Hardy J, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 1999;155:1241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuo A, Akiguchi I, Lee GC, et al. Myelin degeneration in multiple system atrophy detected by unique antibodies. Am J Pathol 1998;153:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya K, Ozawa E, Haga C, et al. Constant involvement of the Betz cells and pyramidal tract in multiple system atrophy: a clinicopathological study of seven autopsy cases. Acta Neuropathol (Berl) 2000;99:628–36. [DOI] [PubMed] [Google Scholar]
- 29.Gray F, Vincent D, Hauw JJ. Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol (Berl) 1988;75:513–18. [DOI] [PubMed] [Google Scholar]
- 30.Benarroch EE, Smithson IL, Low PA, et al. Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann Neurol 1998;43:156–63. [DOI] [PubMed] [Google Scholar]
- 31.Fearnley JM, Lees AJ. Striatonigral degeneration: a clinicopathological study. Brain 1990;113:1823–42. [DOI] [PubMed] [Google Scholar]
- 32.Churchyard A, Donnan GA, Hughes A, et al. Dopa resistance in multiple system atrophy: loss of dopamine D2 receptors. Ann Neurol 1993;34:219–26. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Kusaka H, Matsumoto S, et al. Striatal efferent involvement and its correlation to levodopa efficacy in patients with multiple system atrophy. Neurology 1996;47:1291–9. [DOI] [PubMed] [Google Scholar]
- 34.Wenning GK, Quinn N, Magalhaes M, et al. “Minimal change” multiple system atrophy. Mov Disord 1994;9:161–6. [DOI] [PubMed] [Google Scholar]
- 35.Brooks DJ, Ibanez V, Sawle GV, et al. Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol 1992;31:184–92. [DOI] [PubMed] [Google Scholar]
- 36.Wenning GK, Wagner S, Daniel S, et al. Multiple system atrophy: sporadic or familial? Lancet 1993;342:681. [DOI] [PubMed] [Google Scholar]
- 37.Nee LE, Gomez MR, Dambrosia J, et al. Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin Auton Res 1991;1:9–13. [DOI] [PubMed] [Google Scholar]
- 38.Iwahashi K, Miyatake R, Tsuneoka Y, et al. A novel cytochrome P-450IID6 (CYPIID6) mutant gene associated with multiple system atrophy. J Neurol Neurosurg Psychiatry 1995;58:263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandmann O, Wenning GK, Quinn NP, et al. Arg296 to Cys296 polymorphism in exon 6 of cytochrome P-450-2D6 (CYP2D6) is not associated with multiple system atrophy. J Neurol Neurosurg Psychiatry 1995;59:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairns NJ, Atkinson PF, Kovacs T, et al. Apoprotein E e4 allele frequency in patients with multiple system atrophy. Neurosci Lett 1997;221:161–4. [DOI] [PubMed] [Google Scholar]
- 41.Bandmann O, Sweeney MG, Daniel SE, et al. Multiple-system atrophy is genetically distinct from identified inherited causes of spinocerebellar degeneration. Neurology 1997;49:1598–604. [DOI] [PubMed] [Google Scholar]
- 42.Ozawa T, Takano H, Onodera O, et al. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci Lett 1999;270:110–12. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Forno LS, Di Monte DA, et al. Mutation screening in the α-synuclein gene in MSA. Parkinsonism and Related Disorders 1999;5(suppl):S28. [Google Scholar]
- 44.El-Agnaf OM, Curran MD, Wallace A, et al. Mutation screening in exons 3 and 4 of alpha-synuclein in sporadic Parkinson's and sporadic and familial dementia with Lewy bodies cases. Neuroreport 1998;9:3925–7. [DOI] [PubMed] [Google Scholar]
- 45.Krüger R, Vieira-Saecker AMM, Kuhn W, et al. Increased susceptibility to sporadic Parkinson's disease by a certain combined a-synuclein/apolipoprotein E genotype. Ann Neurol 1999;45:611–17. [DOI] [PubMed] [Google Scholar]
- 46.Engelender S, Kaminsky Z, Guo X, et al. Synphilin-1 associates with a-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 1999;22:110–14. [DOI] [PubMed] [Google Scholar]
- 47.Ostrerova N, Petrucelli L, Farrer M, et al. α-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 1999;19:5782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashida H, Goto J, Zhao N, et al. Cloning and mapping of ZNF231, a novel brain-specific gene encoding neuronal double zinc finger protein whose expression is enhanced in a neurodegenerative disorder, multiple system atrophy (MSA). Genomics 1998;54:50–8. [DOI] [PubMed] [Google Scholar]
- 49.Arima K, Murayama S, Mukoyama M, et al. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 1. Neuronal cytoplasmic inclusions. Acta Neuropathol (Berl) 1992;83:453–60. [DOI] [PubMed] [Google Scholar]
- 50.Murayama S, Arima K, Nakazato Y, et al. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 2. Oligodendroglial cytoplasmic inclusions. Acta Neuropathol (Berl) 1992;84:32–8. [DOI] [PubMed] [Google Scholar]
- 51.Castellani R. Multiple system atrophy: clues from inclusions. Am J Pathol 1998;153:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chin SS, Goldman JE. Glial inclusions in CNS degenerative diseases. J Neuropathol Exp Neurol 1996;55:499–508. [DOI] [PubMed] [Google Scholar]
- 53.Cairns NJ, Atkinson PF, Hanger DP, et al. Tau protein in the glial cytoplasmic inclusions of multiple system atrophy can be distinguished from abnormal tau in Alzheimer's disease. Neurosci Lett 1999;230:49–52. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi K, Miyazu K, Katsukawa K, et al. Cytoskeletal abnormalities in patients with olivopontocerebellar atrophy—an immunocytochemical and Gallyas impregnation study. Neuropathol Appl Neurobiol 1992;18:237–49. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura S, Kawamoto Y, Nakano S, et al. Cyclin-dependent kinase 5 and mitogen-activated protein kinase in glial cytoplasmic inclusions in multiple system atrophy. J Neuropathol Exp Neurol 1998;57:690–8. [DOI] [PubMed] [Google Scholar]
- 56.Gai WP, Power JH, Blumbergs PC, et al. Alpha-synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J Neurochem 1999;73:2093–100. [PubMed] [Google Scholar]
- 57.Head MW, Goldman JE. Small heat shock proteins, the cytoskeleton, and inclusion body formation. Neuropathol Appl Neurobiol 2000;26:304–12. [DOI] [PubMed] [Google Scholar]
- 58.Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci 1998;21:516–20. [DOI] [PubMed] [Google Scholar]
- 59.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett 1994;345:27–32. [DOI] [PubMed] [Google Scholar]
- 60.Weinreb PH, Zhen W, Poon AW, et al. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 1996;35:13709–15. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto M, Hsu LJ, Sisk A, et al. Human recombinant NACP/α-synuclein is aggregated and fibrillated in vitro. Relevance for Lewy body disease. Brain Res 1998;799:301–6. [DOI] [PubMed] [Google Scholar]
- 62.Giasson BI, Uryu K, Trojanowski JQ, et al. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 1999;274:7619–22. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto M, Hsu LJ, Xia Y, et al. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport 1999;10:717–21. [DOI] [PubMed] [Google Scholar]
- 64.Ostrerova-Golts N, Petrucelle L, Hardy J, et al. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 2000;20:6048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dexter DT, Jenner P, Schapira AH, et al. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol 1992;32(suppl):S94–100. [DOI] [PubMed] [Google Scholar]
- 66.Connor JR. Iron acquisition and expression of iron regulatory proteins in the developing brain: manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Dev Neurosci 1994;16:233–47. [DOI] [PubMed] [Google Scholar]
- 67.Duda JE, Giasson BI, Gur TL, et al. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol 2000;59:830–41. [DOI] [PubMed] [Google Scholar]
- 68.Kato S, Shinozawa T, Takikawa M, et al. Midkine, a new neurotrophic factor, is present in glial cytoplasmic inclusions of multiple system atrophy brains. Acta Neuropathol (Berl) 2000;100:481–9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang N, Deuel TF. Pleiotrophin and midkine, a family of mitogenic and angiogenic heparin-binding growth and differentiation factors. Curr Opin Hematol 1999;6:44–50. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Yoshida Y, Goto M, et al. Midkine exists in astrocytes in the early stage of cerebral infarction. Dev Brain Res 1998;106:205–9. [DOI] [PubMed] [Google Scholar]
- 71.Richter-Landsberg C, Gorath M, Trojanowski JQ, et al. Alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res 2000;62:9–14. [DOI] [PubMed] [Google Scholar]
- 72.Muller R, Heinrich M, Heck S, et al. Expression of microtubule-associated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res 1997;288:239–49. [DOI] [PubMed] [Google Scholar]
- 73.Vouyiouklis DA, Brophy PJ. Microtubule-associated proteins in developing oligodendrocytes: transient expression of a MAP2c isoform in oligodendrocyte precursors. J Neurosci Res 1995;42:803–17. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura S, Kawamoto Y, Nakano S, et al. Expression of the endocytosis regulatory proteins Rab5 and Rabaptin-5 in glial cytoplasmic inclusions from brains with multiple system atrophy. Clin Neuropathol 2000;19:51–6. [PubMed] [Google Scholar]
- 75.De la Monte SM, Sohn YK, Ganju N, et al. p53- and CD95-associated apoptosis in neurodegenerative diseases. Lab Invest 1998;78:401–11. [PubMed] [Google Scholar]
- 76.Wullner U, Weller M, Kornhuber J, et al. Altered expression of calcium- and apoptosis-regulating proteins in multiple system atrophy Purkinje cells. Mov Disord 2000;15:269–75. [DOI] [PubMed] [Google Scholar]
- 77.Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 1999;45:421–9. [DOI] [PubMed] [Google Scholar]
- 78.Du C, Dodel RC, Bales KR, et al. Involvement of caspase-3-like cysteine protease in 1-methyl-4-phenylpyridinium-mediated apoptosis of cultured cerebellar granule neurons. J Neurochem 1997;69:1382–8. [DOI] [PubMed] [Google Scholar]
- 79.Hartmann A, Hunot S, Faucheux Y, et al. Is caspase-3 a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease? Parkinsonism and Related Disorders 1999;5(suppl):S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwarz SC, Seufferlein T, Liptay S, et al. Microglial activation in multiple system atrophy: a potential role for NF-kappaB/rel proteins. Neuroreport 1998;9:3029–32. [DOI] [PubMed] [Google Scholar]
- 81.Gerhard A, Banati R, Cagnin A, et al. In vivo imaging of activated microglia with [11C]PK11195 positron emission tomography in patients with multiple system atrophy. Mov Disord 2000;15(suppl 3):215–16. [Google Scholar]
- 82.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control of human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol 2000;63:71–124. [DOI] [PubMed] [Google Scholar]
- 83.Kawamoto Y, Nakamura S, Akiguchi I, et al. Increased brain-derived neurotrophic factor-containing axons in the basal ganglia of patients with multiple system atrophy. J Neuropathol Exp Neurol 1999;58:765–72. [DOI] [PubMed] [Google Scholar]
- 84.Barres BA, Hart IK, Coles HS, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 1992;70:31–46. [DOI] [PubMed] [Google Scholar]
- 85.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signalling molecule in organogenesis and oncogenesis. Neuron 1997;18:847–55. [DOI] [PubMed] [Google Scholar]
- 86.Cannoll PD, Kraemer R, Teng KK, et al. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol Cell Neurosci 1999;13:79–94. [DOI] [PubMed] [Google Scholar]
- 87.Vartanian T, Corfas G, Li Y, et al. A role of the acetylcholine receptor-inducing protein in oligodendrocyte development. Proc Natl Acad Sci U S A 1994;91:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frost EE, Buttery PC, Milner R, et al. Integrins mediate neuronal survival signal for oligodendrocytes. Curr Biol 1999;9:1251–4. [DOI] [PubMed] [Google Scholar]
- 89.Powell SK, Williams CC, Nomizu M, et al. Laminin-like proteins are differentially regulated during cerebellar development and stimulate granule cell neurite outgrowth in vitro. J Neurosci Res 1998;54:233–47. [DOI] [PubMed] [Google Scholar]
- 90.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci 1999;14:199–212. [DOI] [PubMed] [Google Scholar]
- 91.Hagg T, Portera-Cailliau C, Jucker M, et al. Laminins of the adult mammalian CNS; laminin-α2 (Merosin M-) chain immunoreactivity is associated with neuronal processes. Brain Res 1997;764:17–27. [DOI] [PubMed] [Google Scholar]
- 92.Tian M, Hagg T, Denisova N, et al. Laminin-α2 chain-like antigens in CNS dendritic spines. Brain Res 1997;764:28–38. [DOI] [PubMed] [Google Scholar]