Abstract
Background/Aims: CD40, a member of the tumour necrosis factor (TNF) receptor family, is expressed on a variety of haematopoietic cells and is crucial in orchestrating both humoral and cellular immune responses. CD40 is also expressed on some carcinoma cells, where its function remains largely unknown. This study investigated the effects of CD40 ligation on ovarian carcinoma cell growth and apoptosis and on cytokine production, in addition to the role of the NF-κB and JNK signalling pathways.
Methods: CD40 expression was measured in epithelial ovarian carcinoma (EOC) biopsies by immunohistochemistry and in EOC cell lines by flow cytometry. To examine the effects of CD40 ligation on cell growth recombinant soluble CD40 ligand was used to stimulate EOC cell lines and growth was measured by MMT assays. Cytokine production was measured by enzyme linked immunosorbent assays interleukin 8 (IL-8) gene transcription was estimated by means of reverse transcription polymerase chain reaction. The integrity of the CD40 signalling pathway in those cell lines that did not produce cytokines in response to CD40 ligation was assessed by the detection of the transcription factor NF-κB by an electrophoretic mobility shift assay. To investigate the defect in the NF-κB pathway the phosphorylation status of IκBα was determined by an antibody specific to phosphorylated IκBα and dissociation of the IκBα–p65 complex was assessed by co-immunoprecipitation.
Results: CD40 is expressed in primary ovarian carcinoma biopsies and EOC cell lines. CD40 ligation resulted in growth inhibition in most of these carcinoma cell lines and was also found to promote apoptosis, with this last effect only being evident in early passage EOC cells. CD40 ligation also induced significant IL-6 and IL-8 production in most of the EOC cell lines examined and it was confirmed for IL-8 that this effect was regulated at the transcriptional level. NF-κB activation in response to CD40 ligation was found in three of the EOC cell lines and specific defects in the CD40 induced NF-κB pathway were identified in two cell lines. However, CD40 engagement induced JNK activation in all the EOC cell lines.
Conclusions: These data suggest that the CD40 pathway is functional in ovarian carcinoma cells and highlight the need for further studies to provide insight into the role of CD40 in the carcinogenic process and the possible exploitation of this pathway for novel therapeutic approaches.
Keywords: CD40, cytokines, ovarian carcinoma
CD40, a 48 kDa membrane protein belonging to the tumour necrosis factor (TNF) receptor family, is expressed primarily in normal B cells where it regulates growth, survival, and differentiation. CD40 activation in resting B cells provides costimulatory signals for proliferation and isotype switching, enhances survival through upregulation of various antiapoptotic proteins, such as Bcl-2, Bcl-xL, and A20, and induces homotypic cell adhesion and cytokine production.1 The ligand for CD40, CD40L (also known as gp39 or CD154) is a 39 kDa glycoprotein with homology to TNF, which is induced on T cells after their activation via the T cell receptor.2 The CD40–CD40L interaction is central to T cell dependent humoral immune responses and more recent work has shown that the CD40 pathway is also crucial in the generation of cytotoxic T cell responses.2–4 The fundamental importance of the CD40 pathway in immunity is emphasised by studies of X-linked hyper-IgM syndrome (HIGM), a rare immunodeficiency disorder. This condition occurs as a result of an inactivating mutation of CD40L, and is characterised by an inability of B cells to switch immunoglobulin production from IgM to one of the other isotypes in response to presentation of foreign antigen, with consequent deficiency in germinal centre formation.5 Interestingly, a clinically identical syndrome can occur in the presence of normal CD40L as a result of as yet unidentified defects in the CD40 pathway.6 CD40 or CD40L knockout mice have an identical phenotype to that of patients with HIGM.7,8
The biochemical and molecular pathways involved in CD40 function in B cells are not completely understood. CD40 activation in germinal centre B cells induces the activation of protein tyrosine kinases, a phenomenon associated with its antiapoptotic properties in this cell type.9 CD40 signalling in B cells also involves phosphatidylinositol-3-kinase, phosphorylation of phospholipase Cγ2, activation of the c-jun N-terminal kinase (JNK) and p38 stress kinases, and induction of the transcription factor NF-κB.10–14 Because CD40 lacks intrinsic kinase activity, the receptor requires adaptor proteins to transduce its signals. Therefore, CD40 induced JNK and NF-κB signalling is mediated through the interaction of members of the TNF receptor associated factor (TRAF) family—such as TRAF2, TRAF3, TRAF5, and TRAF6—with its cytoplasmic C-terminus.15–17 TRAF2, TRAF3, and TRAF5 interact with the core TRAF binding motif (PxQxT), whereas TRAF6 binds to a membrane proximal region of the CD40 tail.18 The relative contribution the TRAFs to downstream signalling events is complex and evidence supports a role for TRAF2 and TRAF5 in activating the NF-κB inducing kinase (NIK), leading to phosphorylation of components of the IκB kinase complex.19,20 This in turn results in phosphorylation of the IκB proteins, leading to their ubiquitinylation and degradation, with consequent release and nuclear translocation of the p65 subunit of NF-κB.20 Although the pathways regulating TRAF6 induced NF-κB activation remain undefined, this TRAF has been shown to couple CD40 signalling to activation of extracellular signal regulated kinase, thereby regulating cell proliferation.21 Several kinases (for example, ASK1 and GCK1) have been implicated in TRAF induced JNK activation but their role in CD40 activation of the JNK pathway is unknown.22,23
“CD40 activation in germinal centre B cells induces the activation of protein tyrosine kinases, a phenomenon associated with its antiapoptotic properties in this cell type”
Recent work demonstrates that CD40 expression is not limited to cells of haematopoietic origin, but is also present in a variety of other tissue types, including normal endothelial and epithelial cells and some carcinomas.24–26 The restricted expression of CD40 to the proliferative basal compartment of stratified squamous epithelium and the more uniform expression of CD40 on carcinomas suggested a possible role for the CD40 pathway in regulating epithelial cell growth. However, our original work showed that CD40 ligation of carcinoma cell lines resulted in growth inhibition and sensitisation to apoptosis induced by a variety of agents.27 This growth inhibition in response to CD40 activation was also seen in primary keratinocytes and was associated with the induction of terminal differentiation.28,29 These initial studies were mainly performed in the EJ bladder carcinoma cell line, although cell lines derived from different carcinomas (ovarian, cervical, and skin) also responded to CD40 ligation with growth inhibition.27 To investigate whether the original observations are more broadly representative, we have examined the expression and functional consequences of CD40 ligation in epithelial ovarian carcinoma (EOC). CD40 expression was found in both primary EOC biopsies and in carcinoma derived cell lines. The effects of CD40 ligation on ovarian carcinoma cell growth and apoptosis and on cytokine production were analysed, as was the role of the NF-κB and JNK signalling pathways. These data demonstrate that the CD40 pathway is active in carcinoma cells and may control ovarian tumour cell growth, survival, and phenotype.
MATERIALS AND METHODS
Cell culture and isolation of primary EOC cells
Ovarian carcinoma cell lines, OAW-28, SKOV-3, and A2780, were continuously maintained in RPMI supplemented with 10% fetal calf serum (FCS) or DME/10% FCS supplemented with 2 μg/ml bovine insulin and 8 μg/ml ciprofloxacin. The isolation and propagation of the primary ovarian carcinoma cells MG75 and MG79 have been described previously.30 Early passages of these two new lines were used in the experiments described here.
CD40 expression: immunohistochemistry and flow cytometry
To remove paraffin wax, tissue sections from ovarian carcinoma biopsies (Heartlands Hospital, Birmingham) were incubated in xylene (BDH Laboratory Supplies, Poole, Dorset, UK) for 10 minutes with a change of xylene after five minutes, washed with three changes of acetone (BDH), rehydrated by incubating in graded 80%, 50%, and 20% solutions of acetone for one minute each, and then rinsed three times with distilled water. Endogenous peroxidase activity was blocked by incubating the tissue sections in 3% hydrogen peroxide (Sigma, Poole, Dorset, UK)/methanol (BDH) for 15 minutes. The tissue sections were then rinsed with distilled water and subjected to microwave irradiation in a solution of 0.01M citric acid buffer (Sigma), pH 6.0, to retrieve antigens masked by fixation. Briefly, the tissue sections were immersed in 300 ml of citric acid buffer, which was brought to the boil with the microwave set to full power and then boiled for a further five minutes. Evaporated buffer was then replenished and irradiation was continued for a further five minutes. The tissue sections were then cooled under running tap water, ringed with a Dako pen (Dako Ltd, Ely, Cambridgeshire, UK), and placed into coplin jars containing phosphate buffered saline (PBS). Tissue sections were incubated with 50 μl of primary antibody in PBS for one hour (anti-CD40, N-16 at a 1/300 dilution; Santa Cruz Biotechnology, Santa Cruz, San Diego, USA) and washed with gentle agitation in PBS for 10 minutes. Identical sections were also incubated with a control antibody (anti-CD2). The sections were then incubated in 50 μl of a solution containing biotinylated goat antirabbit/mouse (1/100 dilution; Dako)/10% human serum/PBS for 30 minutes. Meanwhile, the streptavidin and biotinylated horseradish peroxidase (HRP) from the Dako HRP ABC kit were complexed in accordance with the manufacturer's instructions. The tissue sections were then washed with gentle agitation in PBS for 10 minutes and incubated in 50 μl of streptavidin–biotinylated HRP complex for 30 minutes. The tissue sections were then thoroughly washed with PBS and antibody binding was visualised by incubation with a substrate solution containing 1 mg/ml diaminobenzidine (Sigma)/3% hydrogen peroxide/Tris buffered saline (TBS), which had been filtered through a 113V filter (Whatman, Maidstone, Kent, UK) before use. Incubation was not allowed to progress for more than 10 minutes to minimise background staining. After visualisation, tissue sections were washed with tap water, counterstained with haematoxylin (Sigma), and mounted with Immunomount (Amersham plc, Little Chalfont, Buckinghamshire, UK). The stained tissue sections were reviewed independently by three investigators who all agreed with the findings. CD40 expression in primary EOC cells isolated from ascites and established cell lines was determined by flow cytometry. Cells were incubated with supernatant from the murine anti-CD40 hybridoma G28.5 or OX34, an anti-CD2 control hybridoma. After incubation with a secondary antimouse IgG–fluorescein isothiocyanate conjugate, the cells were analysed on a FacScan flow cytometer (Coulter, High Wycombe, Buckinghamshire, UK).
MTT incorporation assays
MTT conversion assays were used to measure cell growth as described previously.31 Briefly, cells were plated out at 2–5 × 103 cells/well on 96 well plates and allowed to adhere overnight. The following day they were treated with various concentrations of recombinant soluble CD40L (rsCD40L) (Immunex, Seattle, USA) or vehicle control and incubated for a further 48–72 hours. To determine cell growth, 20 μl of 10 mg/ml MTT (3-(4,4-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma) was added to each well and the plates were incubated at 37°C in 5% CO2 for a further three hours. The supernatant was removed and the formed crystals dissolved in 200 μl dimethyl sulphoxide. The plates were then analysed at 450 nm on a Becton Dickinson plate analyser. Growth inhibition was calculated by expressing the differences in optical densities between treatment wells and control wells as a percentage of the control. Each assay was performed in triplicate. Results from three independent assays were used to calculate the mean differences. Error bars represent standard error of the mean. To assess the effects of combined rsCD40L and cycloheximide (CHX) on EOC cell growth, cells were plated out as described above and then treated for 16 hours with rsCD40L at various concentrations in the presence or absence of 10 μg/ml CHX. All assays were performed in triplicate at least three times and the mean values for each data point calculated from the combined data. To demonstrate that rsCD40L was required for the induction of apoptosis in MG79 cells, the cells were cultured in the presence of rsCD40L, CHX, and increasing concentrations of a neutralising antihuman CD154 monoclonal antibody (24–31; Ancell, Bayport, Minnesota, USA). After incubation for 16 hours, MTT incorporation was estimated as before.
Flow cytometer analysis of cell viability
To distinguish between viable, apoptotic, and necrotic cells a technique using the syto 16 dye was used as reported previously.32 Cells were trypsinised and plated out at a density of 2 × 105/ml in 48 well plates and allowed to attach overnight. After treatment with 1 μg/ml rsCD40L in the presence of 10 μg/ml CHX (Alexis Corporation, San Diego, USA), cells were trypsinised and resuspended in 0.5 ml saline (pre-warmed to 37°C). Syto 16 (Molecular Probes Europe, Leiden, the Netherlands) was added at a concentration of 25nM and incubated with the cells at room temperature for one hour, at which time 5 μg/ml propidium iodide was added. Samples were analysed immediately on a Coulter EPICS XL flow cytometer. A two dimensional dot plot was generated of syto 16 fluorescence versus propidium iodide fluorescence. Syto 16 is only taken up by viable cells and propidium iodide only enters cells whose membranes have become permeabilised; therefore, this technique distinguishes between viable cells (syto 16 positive, propidium iodide negative), apoptotic cells (syto 16 negative, propidium iodide negative), and necrotic cells (syto 16 positive, propidium iodide positive).32 Data for 10 000 cells was collected for each sample and, before data collection, cell debris was excluded by setting a gate on a forward versus side scatter two dimensional dot plot.
ELISA for IL-6, IL-8, and TNF-α
Pelikine Compact™ enzyme linked immunosorbent assay (ELISA) kits (CLB, Amsterdam, the Netherlands) for the detection of each cytokine were purchased from Eurogenetics, UK and the assays performed according to the manufacturer's recommended protocol. Cells were plated on 24 well culture plates at a density of 5 × 104 cells/well and allowed to adhere for 16 hours. The medium was replaced with 2 ml of complete medium with or without rsCD40L (1 μg/ml). The supernatant was collected from stimulated cells and unstimulated controls at various time points. The supernatants were centrifuged to remove cell debris and stored at −70°C until assayed. For each cell line, preliminary assays were performed to determine the optimal dilution at which to assay the supernatants.
IL-8 RT-PCR and Southern blotting
Total RNA was extracted from carcinoma cells and reversed transcribed to cDNA as described. A 550 bp fragment of cDNA spanning exons 3 and 4 of the IL-8 gene was amplified by cycle restricted PCR (25 cycles) using the primers 5`-TGC-AGA-GGG-TTG-TGG-AGA-AG-3` and 5`-CCA-GAC-AAC-ATC-CCA-ACG-GT-3`. The DNA was transferred to Hybond N membrane (Amersham) by Southern blotting as described and detected by hybridisation to a specific probe, 5`-CAG-TGA-AGA-TGC-CAG-TGA-AA-3`, which had been end labelled with γ32P ATP using polynucleotide kinase. The blot was analysed by autoradiography and phosphorimaging. β Actin was amplified from the same cDNAs as described and used as an internal control for total mRNA in each sample.
Electrophoretic mobility shift assays (EMSA)
Treated and control cells were harvested, washed in 500 μl PBS at 4°C and collected by centrifugation. The pellets were lysed in a buffer containing 10mM Hepes (pH 7.9), 1.5mM MgCl2, 10mM KCl, 0.5mM DTT, 0.2% NP40, 1mM phenylmethylsulfonyl fluoride, 10μM leupeptin (Sigma), and 5 μl bovine aprotinin (Sigma) at an approximate concentration of 107 cells/100 μl of buffer. The lysates were microcentrifuged at 16 000 ×g for 10 seconds and the cytoplasmic fraction removed. The nuclear pellet was resuspended in 100 μl of buffer containing 20mM Hepes (pH 7.9), 25% glycerol, 0.42M NaCl 1.5mM MgCl2, 0.5mM DTT, 0.2mM EDTA, and protease inhibitors as before. The lysates were incubated on ice for 10 minutes and then microcentrifuged at 16 000 ×g for 30 seconds. The supernatant was removed and the protein concentration estimated using Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, California, USA). Double stranded oligonucleotides were end labelled using γ32P ATP (Amersham) and T4 polynucleotide kinase (Boehringer, Mannheim, Germany). For the detection of NF-κB binding activity, an oligonucleotide containing the human immunodeficiency long terminal repeat (HIV-LTR) NF-κB consensus binding sequence (5`-GAT-CAG-GGA-CTT-TCC-GCT-GGG-GAC-TTT-CC-3`) was used. In AP-1 experiments, the cyclin D1 AP-1 binding sequence was used (5`-TCC-ATT-CTG-ACT-CAT-TTT-TTT-TAA-3`). Binding reactions containing 10 μg of nuclear protein extract, 1 μg poly dI-dC (Pharmacia, Uppsala, Sweden), 0.1 ng of probe labelled to a specific activity of 2 × 108 counts per minute/μg DNA, and binding buffer containing 4% glycerol, 1mM ETDA, 5mM DTT, 0.01M Tris/HCl (pH 7.5), and 5mM KCl in a total volume of 20 μl were incubated on ice for 30 minutes. For supershift experiments, protein extracts were preincubated with 1 μg of specific monoclonal antibodies (anti-p50, anti-p65, anti-c-Rel; Santa Cruz Biotechnology) before the binding reactions. For competitive inhibition experiments, various concentrations of unlabelled probe were included in the binding reaction. Samples were loaded on to a 5% polyacrylamide gel in 0.5× TBE buffer (5mM Tris/HCl, 0.5 mM EDTA, pH 7.5) and resolved by electrophoresis. The gels were dried and analysed by autoradiography.
Western blotting and immunoprecipitation
After stimulation, cells were lysed in buffer containing 20mM Tris, 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, and 1 μg/ml leupeptin. The lysates were pre-cleared by microcentrifugation at 16 000 ×g for 15 minutes at 4°C. For western blotting, 30 μg of total protein were resolved by sodium dodecyl sulfate polyacrylamide gel electophoresis (SDS-PAGE) and blotted on to a nitrocellulose membrane by standard methods. For the detection of phospho-IκBα, the membranes were incubated in blocking buffer (1× TBS, 0.1% Tween-20, 5% wt/vol skimmed milk powder) at room temperature for one hour. The membranes were then incubated with specific antiphospho-Ser32-IκBα (New England Biolabs, Beverley, Massachusetts, USA), 1/1000 in 1× TBS, 0.05% Tween 20 overnight at 4°C. After washes in 1× TBS, 0.1% Tween, the membranes were incubated with HRP conjugated antirabbit IgG (Sigma), at a 1/2000 dilution, for one hour at room temperature. They were then visualised with enhanced chemiluminescence (ECL; Amersham). Lysates for co-immunopreciptation experiments were performed as described above. After pre-clearance, 200 μg of total protein was incubated with 2 μl of anti-p65 monoclonal antibody (Santa Cruz) for three hours at 4°C. Immune complexes were captured on protein G sepharose beads. Following washes, 50 μl gel sample buffer was added and the complexes heated to 95°C for five minutes. They were then resolved by SDS-PAGE and transferred to nitrocellulose membrane. The complexes were detected by incubating the membrane with an anti-IκBα antibody (Santa Cruz) overnight at 4°C. The membrane was washed and incubated with an HRP conjugated antirabbit IgG and visualised with ECL.
In vitro kinase assay for JNK activity
Cells were grown to 80% confluence in monolayer culture on 6 cm2 culture plates. Stimulation was carried out by the addition of rsCD40L, to a final concentration of 1 μg/ml, or vehicle control. At 15 minutes, the plates were washed twice with ice cold PBS and lysed on ice for 15 minutes in 500 μl lysis buffer (20mM Tris/HCl, pH 7.6, 0.5% Triton X-100, 250mM NaCl, 3mM EGTA, 3mM EDTA) to which were added 2mM PMSF, 2mM sodium vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1mM DTT. The lysates were microcentrifuged for 15 minutes at 16 000 ×g (4°C). Active JNK was immunoprecipitated from 200 μg of total protein by incubation with 0.4 μg anti-JNK antibody (Santa Cruz) at 4°C for three hours. Immune complexes were retrieved by the addition of 40 μl of protein G sepharose beads (Pharmacia) in a 50% slurry and incubated for a further two hours. The beads were washed in lysis buffer, followed by two further washes in kinase assay buffer (20mM Hepes, pH 7.5, 20mM β-glycerophosphate, 10mM MgCl2, 10mM MnCl2, 1mM DTT, 50μM sodium vanadate). The beads were resuspended in 30 μl kinase assay buffer containing 2 μg GST–Jun (Santa Cruz) and 1 μCi γ32P ATP. The samples were incubated for 30 minutes at 30°C and the kinase reactions terminated by the addition of 30 μl sample buffer. The samples were heated to 95°C for five minutes and the supernatants were resolved by SDS-PAGE. The dried gels were analysed by autoradiography and phosphorimaging.
RESULTS
CD40 expression in primary EOCs and ovarian carcinoma cell lines
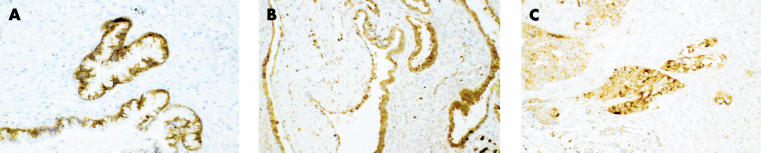
CD40 expression in primary EOCs was assessed by immunohistochemistry using a rabbit polyclonal antibody raised against an N-terminal epitope of CD40 (N-16; Santa Cruz). In total, 20 paraffin wax embedded sections of ovarian tumour representing a variety of histological subtypes were analysed. CD40 expression was evident in the epithelial elements of all the tumours examined. Figure 1 ▶ shows three examples representing the spectrum of histological abnormality found in ovarian neoplasia. Cases of borderline ovarian carcinoma displayed a uniformly dense CD40 staining of the epithelial cells, whereas the stroma was negative (fig 1A ▶). Well differentiated ovarian tumours also expressed CD40 in the epithelial cell component (fig 1B ▶), whereas a more punctate pattern of CD40 expression was seen in poorly differentiated tumours (fig 1C ▶).
Figure 1.
CD40 is expressed in ovarian carcinoma. Paraffin wax embedded ovarian carcinoma tissue was stained by immunochistochemistry using a rabbit polyclonal antibody raised against an N-terminal epitope of CD40 (N-16). Staining of three representative tumours is shown: (A) a borderline tumour, (B) a well differentiated serous cystadenocarcinoma, and (C) an undifferentiated tumour.
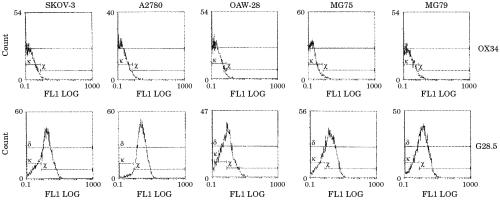
CD40 expression in EOC tumour cell lines was determined by flow cytometry using the G28.5 monoclonal antibody. Three long established (OAW-28, A2780, and SKOV-3) and two early passage ovarian tumour cell lines isolated from ascites (MG75 and MG79) were analysed and all were found to express high amounts of CD40 (fig 2 ▶).
Figure 2.
Expression of CD40 by cultured ovarian carcinoma cells was determined by flow cytometry.
The effects of CD40 ligation on ovarian tumour cell growth and survival
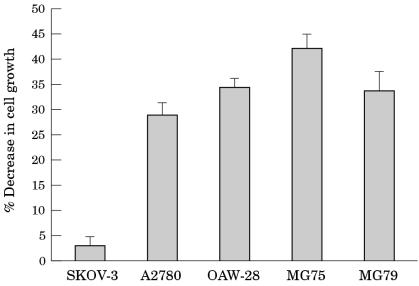
The expression of CD40 on ovarian carcinomas prompted us to examine the effects of activation of this pathway on cell growth. For this purpose we used rsCD40L, a trimeric molecule that mimics the structure of CD40L expressed on the surface of activated T cells. EOC cell lines were treated with 1 μg/ml rsCD40L and cell growth was determined 72 hours later using MTT assays. A significant reduction in growth of OAW-28, A2780, MG75, and MG79 but not of SKOV-3 cells was seen (fig 3 ▶). Flow cytometric analysis of DNA content in A2780 and OAW-28 cells was performed and showed that CD40 induced growth inhibition does not involve an obvious growth arrest at a particular point in the cell cycle (data not shown). In addition, CD40L treated ovarian carcinoma cells showed no morphological evidence of apoptotic cell death, as assessed by phase contrast microscopy, suggesting that the observed decrease in proliferation does not result from a reduction in viability.
Figure 3.
CD40 ligation inhibits the growth of ovarian carcinoma cell lines. Epithelial ovarian carcinoma (EOC) cell lines were treated for 72 hours with 1 μg/ml recombinant soluble CD40 ligand (rsCD40L) and cell growth was assessed by MTT assay. The data are representative of three independent experiments and the values are presented as the mean (± SD) of triplicate determinations.
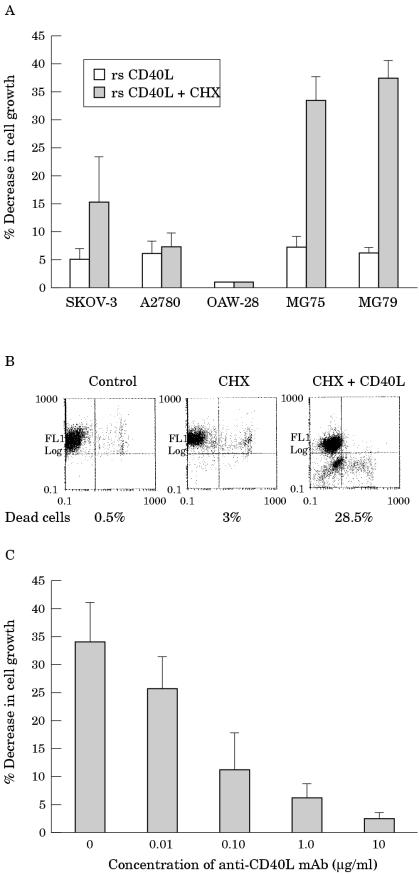
Inhibition of de novo protein synthesis has been shown to be required for the induction of apoptosis by TNF-α or anti-Fas monoclonal antibody treatment in several established cell lines.33,34 To determine whether a similar requirement exists with respect to CD40 activation, EOC cell lines were exposed to 1 μg/ml rsCD40L in the presence or absence of 10 μg/ml of the protein synthesis inhibitor CHX, and cell growth was assessed 16 hours later by MTT assays. Under these conditions, it was found that CHX treatment had little or no impact on the effects of CD40 ligation on SKOV-3 and A2780 cell growth (fig 4A ▶). It was not possible to examine the response of OAW-28 cells to the CHX/rsCD40L combination because CHX alone induced a dramatic cytotoxicity in this cell line, even at very low concentrations (1–5 μg/ml). Interestingly, stimulation of the early passage MG75 and MG79 ovarian carcinoma cells with rsCD40L in the presence of CHX resulted in a significant reduction in cell growth within 16 hours (fig 4A ▶). Exposure to rsCD40L alone for the same period of time induced only a small inhibition of proliferation in MG75 and MG79. The effect of CHX and rsCD40L combination treatment on MG75 and MG79 cell growth was the result of induction of apoptosis, as determined by morphological criteria using phase contrast microscopy and propidium iodide staining for the detection of condensed and degraded nuclei, characteristic of apoptotic cells (data not shown). To confirm that CD40 ligation in the presence of CHX induced apoptosis, we used flow cytometric analysis with two fluorescent stains, syto 16 and propidium iodide, which allows quantitative assessment of both apoptosis and necrosis. The propidium iodide assay verified that rsCD40L treatment alone does not affect survival but induces apoptosis upon inhibition of protein synthesis in early passage MG79 carcinoma cells (fig 4B ▶). To establish that the observed phenomenon is dependent on CD40 activation, MG75 cells were incubated with CHX and rsCD40L in the presence of increasing concentrations of neutralising anti-CD40L monoclonal antibody. As shown in fig 4C ▶, this monoclonal antibody significantly reversed the apoptosis inducing effect of CHX/rsCD40L treatment in a dose dependent manner.
Figure 4.
CD40 ligation induces apoptosis in epithelial ovarian carcinoma (EOC) cell lines in the presence of cycloheximide (CHX). (A) EOC cells were treated for 16 hours with 1 μg/ml recombinant soluble CD40 ligand (rsCD40L) in the presence or absence of 10 μg/ml CHX and cell growth was assessed using MTT assays. The results of rsCD40L and CHX treatment were compared with CHX treatment alone to calculate the effects on cell growth. In the presence of CHX, CD40 stimulation induced a significant decrease in cell growth in MG75 and MG79 early passage EOC cell lines. Data shown represent the mean value (± SD) of triplicate determinations and are representative of at least three independent experiments. (B) Flow cytometric analysis using syto 16 and propidium iodide confirmed that the treatment of MG79 cells with rsCD40L and CHX resulted in cell death and that this was predominantly via apoptosis (panel 3; syto 16 negative and propidium iodide negative), although necrotic cell death was also observed (panel 4; syto 16 negative and propidium iodide positive). Note that the syto 16 fluorescence is depicted on the y axis and the propidium iodide fluorescence is on the x axis. (C) The specificity of CD40 mediated cell death in MG79 cells is demonstrated by the ability of a neutralising anti-CD40L antibody (mAb) to block the effects of combination treatment with rsCD40L and CHX. MG79 cells were exposed to 1 μg/ml rsCD40L and 10 μg/ml CHX in the presence or absence of increasing concentrations of antihuman CD40L antibody (clone 24-31) and cell growth was assessed 16 hours later using MTT assays. Data shown represent the mean value (± SD) of triplicate determinations and are representative of three independent experiments.
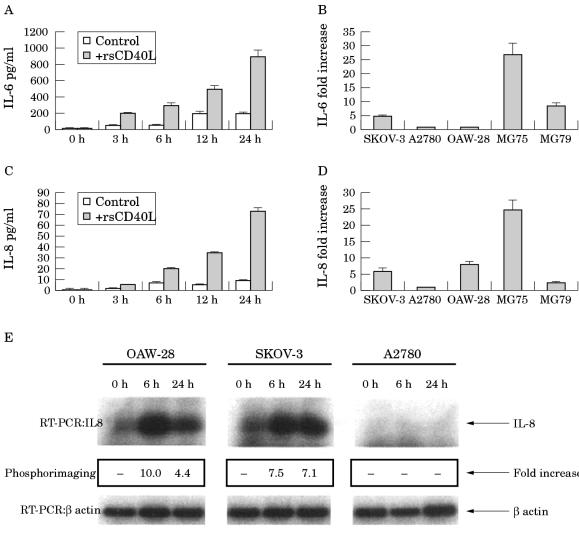
CD40 activation results in enhanced IL-6 and IL-8 but not TNF-α production by ovarian carcinoma cell lines
Because CD40 ligation results in cytokine induction in B cells and certain carcinoma cell lines, we investigated the ability of rsCD40L treatment to induce cytokine production in EOC cell lines. Interleukin 6 (IL-6) and TNF-α were selected for analysis because these cytokines are frequently expressed in the normal and malignant ovarian epithelium and recent data indicate that IL-8 can be induced in EOC cells by various treatments.35–37 SKOV-3 cells were first treated with 1 μg/ml rsCD40L or vehicle control and supernatants were collected at various time intervals (three, six, 12, and 24 hours) for IL-6 determination. Although SKOV-3 cells secreted basal amounts of this cytokine, IL-6 production was significantly increased in the presence of rsCD40L in a time dependent manner. Thus, IL-6 concentrations increased 4.5 fold after 24 hours of treatment with rsCD40L (fig 5A ▶). The effects of CD40 ligation on IL-6 production in other EOC cell lines after 24 hours of treatment was then determined. As with SKOV-3 cells, treatment with 1 μg/ml rsCD40L increased IL-6 values 10 fold in MG79 cells and 26 fold in MG75 cells (fig 5B ▶). However, A2780 and OAW-28 cells secreted no basal IL-6 and IL-6 was not induced following rsCD40L treatment (fig 5B ▶).
Figure 5.
CD40 ligation modulates interleukin (IL) production in epithelial ovarian carcinoma (EOC) cell lines. (A) Time course of recombinant soluble CD40 ligand (rsCD40L) stimulated IL-6 secretion in the SKOV-3 cell line. SKOV-3 cells were treated with 1 μg/ml rsCD40L for various time intervals (zero, three, six, 12, or 24 hours) and conditioned supernatants were analysed for IL-6 concentrations using an IL-6 specific enzyme linked immunosorbent assay (ELISA). Cytokine production from control untreated cells is also shown. Data shown represent the mean value (± SD) of triplicate determinations and are representative of at least three independent experiments. (B) CD40 stimulation induces IL-6 secretion in some but not all the EOC cell lines. SKOV-3, MG75, and MG79 cells produced appreciable amounts of IL-6 after a 24 hour exposure to 1 μg/ml rsCD40L, but A2780 and OAW-28 cells failed to respond. Results are depicted as fold increase in IL-6 production and are the mean values (± SD) of three independent experiments. (C) Time course of rsCD40L stimulated IL-8 secretion in the OAW-28 cell line. OAW-28 cells were treated with 1 μg/ml rsCD40L for various time intervals (zero, three, six, 12, or 24 hours) and conditioned supernatants were analysed for IL-8 concentrations using an IL-8 specific ELISA. Cytokine production from control untreated cells is also shown. Data shown represent the mean value (± SD) of triplicate determinations and are representative of at least three independent experiments. (D) CD40 stimulation induces IL-8 secretion in most but not all the EOC cell lines. SKOV-3, OAW-28, MG75, and MG79 cells produced appreciable amounts of IL-8 after a 24 hour exposure to 1 μg/ml rsCD40L but A2780 cells failed to respond. Results are depicted as fold increase in IL-8 production and are the mean values (± SD) of three independent experiments. (E) CD40 ligation induces increased IL-8 transcription. Cell lines were stimulated with rsCD40L (1 μg/ml) and collected at the indicated time points. Total cellular RNA was extracted and reverse transcribed as described. Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was performed for 25 cycles with specific primers for a 500 bp fragment of the IL-8 cDNA or β actin. The amplified DNA was transferred by Southern blotting and quantified as described. Although rsCD40L induces a pronounced increase in IL-8 mRNA in OAW-28 and SKOV-3 cells, no such effect is seen in A2780.
Basal IL-8 was detected in the supernatant from cultures of four of the five lines tested (SKOV-3, OAW-28, MG75, and MG79). A time dependent increase in CD40L induced IL-8 secretion was seen in the OAW-28 cell line, resulting in an eightfold induction after 24 hours (fig 5C ▶). Examination of IL-8 values in other EOC cell lines exposed to rsCD40L for 24 hours also showed a significant increase in cytokine secretion (fig 5D ▶). Thus, IL-8 concentrations were increased sixfold in SKOV-3 cells, 25 fold in MG75 cells, and 1.8 fold in MG79 cells. However, no IL-8 was detected in the supernatant of A2780 cells and even prolonged incubations (48–72 hours) with rsCD40L failed to induce IL-8. In contrast to IL-6 and IL-8, CD40 activation failed to induce TNF-α secretion in any of the EOC cell lines tested (data not shown).
To determine whether the effect of CD40 ligation on IL-8 secretion resulted from increased gene transcription, cycle restricted reverse transcription polymerase chain reaction (RT-PCR) was performed on RNA extracted from control and CD40L treated A2780, OAW-28, and SKOV-3 cells. Some basal IL-8 transcription was detected in OAW-28 and SKOV-3 cells but not in A2780 cells (fig 5E ▶). In accordance with the IL-8 secretion data, there was a pronounced increase in IL-8 transcription (up to 10 fold) in OAW-28 and SKOV-3 cells after stimulation with rsCD40L, but no effect in A2780 cells. These experiments confirm that the CD40 pathway regulates IL-8 at the transcriptional level.
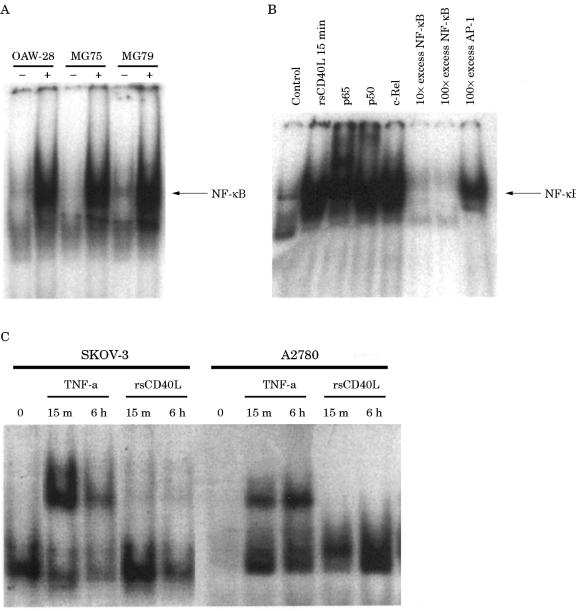
CD40 ligation activates NF-κB in EOC cells
The inability of CD40 activation to induce cytokine production in certain ovarian carcinoma cell lines prompted us to examine the integrity of the CD40 signalling pathway in these cells. For this purpose, we first examined the effects of rsCD40L on NF-κB activation by EMSAs using a standard oligonucleotide probe containing the HIV-LTR NF-κB consensus. Time course experiments established that NF-κB activity was undetectable in unstimulated EOC cells but that activation occurred as early as 15 minutes after CD40 ligation with 1 μg/ml rsCD40L, peaked at between three and six hours, and remained raised for at least 24 hours after stimulation (data not shown). Experiments were then performed to determine NF-κB activation in all five EOC cell lines using a single three hour time point. As shown in fig 6A ▶, little or no basal NF-κB binding activity was found in unstimulated cells. However, in the presence of rsCD40L a significant increase in NF-κB binding was observed in OAW-28, MG75, and MG79 cells (fig 6A ▶). The specificity of this protein–DNA interaction was confirmed by inhibition with an excess of unlabelled NF-κB probe, but not with excess AP-1 probe, in MG79 cells (fig 6B ▶). To determine the composition of the CD40 induced NF-κB complexes, specific antibodies against the p50, p65, and c-Rel subunits were used. Exposure of nuclear extracts from rsCD40L treated MG79 cells to anti-p50 and anti-p65 antibodies supershifted these complexes, whereas the anti-c-Rel antibody had no effect (fig 6B ▶), suggesting that the bound NF-κB contains p50–p65 heterodimers.
Figure 6.
CD40 ligation activates NF-κB in epithelial ovarian carcinoma (EOC) cells. The effects of CD40 activation on NF-κB binding activity were determined by an electrophoretic mobility shift assay (EMSA). Cells were stimulated for three hours with 1 μg/ml recombinant soluble CD40 ligand (rsCD40L), subsequently lysed, and nuclear extracts prepared as described earlier. In three of the five cell lines tested (OAW-28, MG75, and MG79) there was a rapid and sustained induction of NF-κB binding activity, which was maximal at three to six hours (A) and persisted for up to 24 hours (data not shown). Supershift analysis demonstrated that the bound complex in MG79 cells consisted of p50–p65 heterodimers but not c-Rel (B). The specificity of the binding activity was demonstrated by co-incubating the DNA bound NF-κB with excess unlabelled oligonucleotide. There is a loss of binding to labelled probe in the presence of excess unlabelled NF-κB oligonucleotide, but binding still occurs in the presence of a 100 times excess of the irrelevant probe (B). In the SKOV-3 and A2780 cell lines, stimulation with rsCD40L failed to activate NF-κB, whereas treatment with 50 ng/ml tumour necrosis factor α (TNF-α) induced NF-κB binding in both of these cell lines (C).
Interestingly, CD40 ligation in SKOV-3 and A2780 cells failed to induce NF-κB binding activity above basal values (fig 6C ▶). Extensive time course experiments were also performed over various time intervals (15 minutes to 24 hours) and also failed to detect an increase in CD40 induced NF-κB binding activity in these cells. To determine whether the NF-κB pathway is intact in these two lines with respect to other stimuli, EMSAs were performed on nuclear extracts from the cell lines stimulated with TNF-α, a potent inducer of NF-κB activation in several cell systems. These experiments revealed that NF-κB activity was induced by TNF-α in both SKOV-3 and A2780 cell lines (fig 6C ▶). Thus, A2780 and SKOV-3 cells appear to be selectively defective in CD40 induced NF-κB activation but not in TNF-α induced activation.
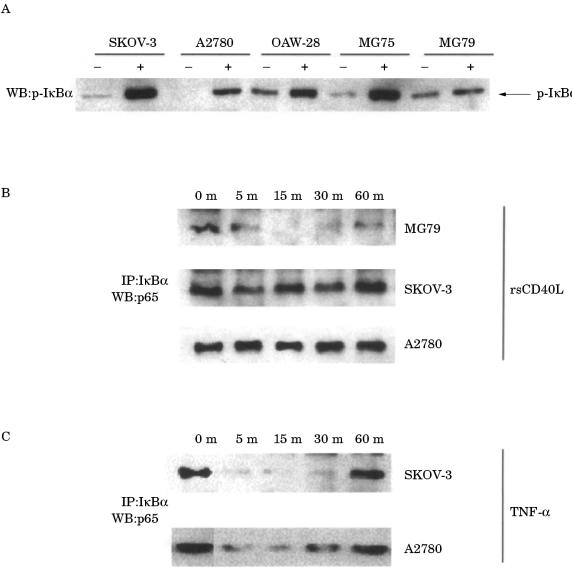
CD40 ligation induces phosphorylation of IκBα and nuclear translocation of p65
To identify the defect in the CD40 induced NF-κB pathway in SKOV-3 and A2780 cells, we first determined the status of IκBα phosphorylation in response to CD40 activation in these cell lines using an antibody specific to IκBα phosphorylated at the serine 32 residue. IκBα was phosphorylated within five minutes of stimulation with rsCD40L in all the EOC cell lines, including SKOV-3 and A2780, where induction of NF-κB binding activity was absent (fig 7A ▶). Because phosphorylation of IκBα alone is not sufficient to cause dissociation of IκBα from p65, co-immunoprecipitation experiments were performed to determine whether phosphorylated IκBα dissociated from p65 after stimulation with rsCD40L. Treatment with rsCD40L resulted in rapid dissociation of IκBα from p65 in MG79 cells (fig 7B ▶). However, no such effect was seen in the rsCD40L treated SKOV-3 and A2780 cell lines, where the IκBα complex with p65 remained largely intact; only a marginal dissociation at five minutes could be detected (fig 7B ▶). However, stimulation of SKOV-3 and A2780 cells with TNF-α resulted in rapid dissociation of IκBα from p65, consistent with its ability to induce NF-κB binding activity in these cell lines (fig 7C ▶).
Figure 7.
CD40 ligation induces IκBα phosphorylation in epithelial ovarian carcinoma (EOC) cell lines but fails to promote dissociation of IκBα from p65 in SKOV-3 cells. (A) The phosphorylation status of IκBα was determined by immunoblot analysis using a specific antiphospho-IκBα Ser32 polyclonal antibody (New England Biolabs, USA). Rapid phosphorylation occurred in all five cell lines on stimulation with 1 μg/ml recombinant soluble CD40 ligand (rsCD40L) (+) as compared with vehicle control (−). (B) Co-immunoprecipitation experiments were performed to determine whether IκBα remained bound to p65 in EOC cells following CD40 activation. Complexes were immunoprecipitated with an anti-p65 monoclonal antibody (sc-7151). After immunoblotting, the membranes were probed with an anti-IκBα polyclonal antibody (sc-203-G). Treatment with rsCD40L induced rapid dissociation of IκB-α from p65 in MG79 cells but not in SKOV-3 or A2780 cells. (C) Tumour necrosis factor α (TNF-α) was able to induce IκBα dissociation from p65 in SKOV-3 and A2780 cells.
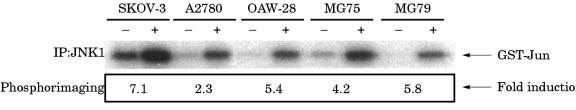
CD40 stimulation activates the JNK/AP-1 cascade in EOC cell lines
The JNK (also known as the stress activated protein kinase; SAPK) signalling cascade is initiated upon stimulation of various members of the TNF receptor superfamily—including TNFRI, TNFRII, CD40, and Fas—and leads to phosphorylation and activation of the AP-1 transcription factor c-Jun. Transactivation of both the IL-6 and IL-8 genes requires AP-1 binding activity and therefore we investigated whether or not the AP-1 pathway was activated in these cells in response to CD40 ligation. Immune complex kinase assays were performed on extracts from EOC cells treated with 1 μg/ml rsCD40L, using GST–cJun(1–79) as a substrate. Initial time course experiments in SKOV-3 and OAW-28 cells revealed a transient increase in JNK activity—the degree of c-Jun phosphorylation increased within five minutes of stimulation, peaked at 15 minutes, and returned to basal values by 60 minutes (data not shown). Thus, JNK activity was measured 15 minutes after CD40 ligation in all EOC cell lines and increases varying from 2.3 fold to 7.1 fold compared with basal values were observed (fig 8 ▶). Activation of JNK was associated with increased binding to an oligonucleotide probe containing an AP-1 concensus sequence derived from the cyclin D1 promoter as determined by EMSA (data not shown).
Figure 8.
CD40 ligation stimulates c-Jun N-terminal kinase (JNK) activation in epithelial ovarian carcinoma (EOC) cell lines. Cells were treated for 15 minutes with 1 μg/ml recombinant soluble CD40 ligand (rsCD40L) and JNK activity was assessed by immune complex kinase assays using GST–cJun(1–79) as substrate. A representative assay is shown. Relative degrees of JNK activation were quantitated on a phosphorimager and are shown as fold increase. At least three independent experiments were performed and gave similar results.
DISCUSSION
Our present study extends our previous work demonstrating that ligation of CD40 on epithelial cells results in growth inhibition and sensitisation to apoptosis.27 We show that CD40 is expressed in epithelial ovarian carcinoma cell lines in addition to primary tumours. Ligation of CD40 on ovarian carcinoma cell lines resulted in growth inhibition and apoptosis in response to treatment with a protein synthesis inhibitor. This is in keeping with the effects seen in HeLa cells stably transfected with CD40,38,39 but is contrary to that observed in normal B cells, where activation of the CD40 pathway provides a potent survival and proliferative stimulus.40 Other members of the TNF receptor family have also been shown either to promote cell growth or induce apoptosis, dependent on cell type, and recent work in B cell lines suggests that the differentiation status of the cell may influence the outcome of CD40 activation.41–43 Thus, the growth of B cell lymphoma cell lines both in vitro and in vivo can be inhibited by CD40 ligation.44 Our work with Burkitt's lymphoma cell lines also demonstrates that CD40 activation induces growth arrest but, contrary to epithelial cells, this effect results in protection from apoptosis.41 Although most of the ovarian carcinoma cell lines examined responded to prolonged CD40 ligand exposure with growth inhibition, only two of the cell lines displayed apoptosis when treated for a shorter period with CD40L in the presence of the protein synthesis inhibitor, CHX (table 1 ▶). This apoptosis induction was most pronounced in the recently established early passage ovarian carcinoma cell lines (MG75 and MG79), suggesting that adaptation to long term cell culture may select for cells that are relatively resistant to CD40 induced apoptosis. The differential response of the carcinoma cell lines to either growth inhibition or apoptosis suggests that different signalling pathways may be responsible for these effects. In this regard, the absence of a death domain in the CD40 cytoplasmic tail implicates other transduction pathways in apoptosis induction, such as those mediating the activation of JNK.
Table 1.
Summary of the effects of CD40 ligation on the panel of ovarian carcinoma cell lines
| Growth inhibition | Apoptosis induction | IL-6 production | IL-8 production | NF-κB activation | JNK activation | |
| SKOV-3 | − | − | + | + | − | + |
| A2780 | + | − | − | − | − | + |
| OAW-28 | + | − | − | + | + | + |
| MG75 | + | + | + | + | + | + |
| MG79 | + | + | + | + | + | + |
The induction of apoptosis was determined by recombinant soluble CD40 ligand treatment in the presence of cycloheximide (CHX). Note that the OAW-28 cell line was extremely sensitive to CHX alone and that this precluded determination of the effect of CD40 ligation. IL, interleukin; JNK, c-jun N-terminal kinase.
“Our study demonstrates, for the first time, that CD40 ligation can induce IL-8 production from ovarian carcinoma cells”
Secretion of both IL-6 and IL-8 was stimulated by CD40 ligation in some of the ovarian carcinoma cell lines (table 1 ▶). The effect of the CD40 pathway on IL-6 production from epithelial cells has been described previously.45,46 Production of IL-6 by ovarian carcinoma cells has also been described.35,37 Our study also demonstrates, for the first time, that CD40 ligation can induce IL-8 production from ovarian carcinoma cells. This cytokine is a potent chemotactic agent that can also function to promote carcinoma cell growth and angiogenesis.47 Previous studies have shown that IL-8 secretion can be induced in primary epithelial cells and in carcinoma cell lines by a variety of diverse stimuli including TNF-α, amoebiasis, and Helicobacter pylori.48,49 One study demonstrated that ovarian carcinoma cell lines and fresh tumour explants can spontaneously secrete IL-8 and that this can be further induced by treatment with paclitaxel (Taxol), an anti-neoplastic drug.36 Thus, the proinflammatory IL-6 and IL-8 cytokines that are present in the malignant ascites of patients with ovarian carcinoma can be induced by CD40. This suggests that the pathways mediating the expression and secretion of these cytokines are active in vivo and perhaps involve endogenous stimulation of the CD40 pathway.
CD40 ligation has been shown to result in the activation of the NF-κB transcription factor in a variety of different cell types, including epithelial cells.46 Accordingly, CD40 stimulation induced NF-κB DNA binding activity in three of the five ovarian carcinoma cell lines examined; interestingly, the two newly established cell lines gave the most robust activation of NF-κB (table 1 ▶). Although CD40 ligation did not significantly activate NF-κB in the SKOV-3 and A2780 cell lines, the treatment of these cell lines with TNF-α resulted in activation of the NF-κB pathway. Given that the cell signalling pathways responsible for NF-κB activation by CD40 and TNF-α involve the TRAF2 signal transducer molecule, a specific defect in CD40 induction of NF-κB activity is intriguing.50 The activation of NF-κB is regulated by cytoplasmic retention through binding to IκB proteins.20 Signals that activate NF-κB initially result in the degradation of IκB, thereby permitting the NF-κB complex to translocate to the nucleus.20 The degradation of IκBα is induced by phosphorylation at two serine residues in the N-terminus of the molecule (S32 and S36), resulting in ubiquitin mediated proteasomal degradation.20,51 CD40L treatment was able to induce the rapid phosphorylation of IκBα, as measured using a monoclonal antibody specific for S32, in all the ovarian carcinoma cell lines, including those that were unable to activate NF-κB in response to this stimulus. Further examination revealed that although phosphorylated IκBα could readily dissociate from the NF-κB complex in response to CD40 ligation in those ovarian carcinoma cell lines where NF-κB activation was observed, this was not the case in the cell lines (SKOV-3 and A2780) with a defective NF-κB response. Interestingly, even though CD40 ligation was unable to induce the dissociation of IκBα from p65 in the SKOV-3 and A2780 cell lines, TNF-α stimulation resulted in efficient dissociation of IκBα with concomitant activation of NF-κB in these cell lines. Thus, there appears to be a specific defect in the CD40 pathway in the SKOV-3 and A2780 cell lines, which results in a failure of IκBα to dissociate from p65 in response to receptor activation. This could result from defective phosphorylation of IκBα at sites other than S32 that have been shown to be important for degradation, such as S36.20,51 In this context, the demonstration that the DNA dependent protein kinase inhibits NF-κB activity by phosphorylating IκBα at S36 and at threonine residue 273 in the C-terminus of the protein is of interest, and indicates that differential phosphorylation is required for full activation of NF-κB.52 Furthermore, the inducible IκB kinases (IKKs) are also involved in the phosphorylation of IκBα in response to TNF-α and are thus likely to be responsible for mediating CD40 induced NF-κB activation.20,53 A differential effect of CD40 versus TNF-α on IKK mediated phosphorylation of IκBα is possible given recent data demonstrating that the IKK complex can transduce distinct signals via IKKα or IKKβ, dependent on the nature of the stimulus.54 Thus, the regulation of NF-κB activity through IκBα is extremely complex and the ovarian carcinoma cell lines used in our study may help to dissect these pathways.
“Microtubule interfering agents activate JNK in ovarian carcinoma cell lines”
Specific defects resulting in the loss of functional IκBα have been demonstrated in Hodgkin's disease cell lines, suggesting a contribution of deregulated NF-κB activity to the aetiology of this malignancy.55 However, a role for the specific defect in NF-κB activation described here in the pathogenesis of ovarian carcinoma is unlikely. The two newly established ovarian carcinoma cell lines were able to activate NF-κB in response to CD40 ligation and the NF-κB defect in SKOV-3 and A2780 cell lines did not correlate with the ability of these cells to respond to CD40L treatment with growth inhibition, apoptosis, or cytokine secretion. Paradoxically, although CD40 activation resulted in minimal induction of NF-κB binding activity in the SKOV-3 cell line, it did result in increases in IL-6 and IL-8 production, although not to the same degree as the MG75 and MG79 cell lines (table 1 ▶). Transactivation of these genes has been shown to require NF-κB, and our data using both IL-8 reporter mutants or dominant negative mutants (NJ Gallagher et al, 2000, unpublished observations) that block either the NF-κB or JNK pathway demonstrate that NF-κB activation is an absolute requirement for CD40 induced IL-8 transcription.56,57 It is possible that low level NF-κB activation in SKOV-3 cells is sufficient to account for the induction of IL-8. Interestingly, CD40 ligation in the OAW-28 and MG79 cells resulted in robust activation of the NF-κB pathway although only inducing IL-8 values similar to those observed in SKOV-3 cells. This serves to emphasise the contribution of other signalling pathways to CD40 mediated cytokine production. Activation of the JNK stress kinase pathway in response to CD40 ligation has been demonstrated previously in B cell lines.11,12 The AP-1 transcription factor family is also required for full transactivation of the IL-6 and IL-8 genes. We have now shown that this pathway is also activated in response to CD40 stimulation in ovarian carcinoma cells and that the JNK pathway is required via AP-1 for the full activation of IL-8 transcription. CD40 ligation induced JNK activation in all the EOC cell lines examined, irrespective of their ability to respond to CD40L treatment with NF-κB activation, growth inhibition, apoptosis, or cytokine secretion.
Future work will investigate the specific contribution of the JNK and associated p38 kinase pathways to CD40 induced effects. Previous work has shown that microtubule interfering agents activate JNK in ovarian carcinoma cell lines, and this pathway has been implicated in the apoptotic response of carcinoma cells to cis-platin.58–60 Combined cis-platin and paclitaxel, a microtubule interfering agent, are the first line therapy for ovarian carcinoma, and the ability of CD40 ligation to activate the JNK pathway and to sensitise carcinoma cells to apoptosis induced by cytotoxic agents may thus be of therapeutic relevance. In this context, we have found a significant survival benefit of CD40L treatment alone in severe combined immunodeficient mice bearing human breast carcinoma cells,61 and recent data indicate that CD40L treatment may result in indirect effects on anti-tumour immune responses, in addition to direct effects on tumour growth.62 Thus, further dissection of the cell signalling pathways mediating the effects of CD40 ligation in ovarian carcinoma cells will be relevant to both an understanding of the role of these pathways in the carcinogenic process and to the possible development of more effective treatments.
Take home messages.
The CD40 pathway appears to be is functional in ovarian carcinoma cells
Further studies are needed to provide insight into the role of CD40 in the carcinogenic process
This may lead to the possible exploitation of this pathway for novel therapeutic approaches
Acknowledgments
We are grateful to Immunex Corporation for the gift of soluble trimeric CD40L. This work was supported by the Cancer Research Campaign, Medical Research Council, and by an Albert McMaster and Helen Tompkinson Award for Cancer Research granted to NJG by the British Medical Association.
Abbreviations
CD40L, CD40 ligand
CHX, cycloheximide
ECL, enhanced chemiluminescence
ELISA, enzyme linked immunosorbent assay
EMSA, electrophoretic mobility shift assay
EOC, epithelial ovarian carcinoma
FCS, fetal calf serum
HIGM, X-linked hyper-IgM syndrome
HIV-LTR, human immunodeficiency virus long terminal repeat
HRP, horseradish peroxidase
IKK, inducible IκB kinase
IL, interleukin
JNK, c-jun N-terminal kinase
NIK, NF-κB inducing kinase
PBS, phosphate buffered saline
rsCD40, recombinant soluble CD40 ligand
RT-PCR, reverse transcription polymerase chain reaction
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
TBS, Tris buffered saline
TNF, tumour necrosis factor
TRAF, TNF receptor associated factor
REFERENCES
- 1.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand–receptor pair for thymus-dependent B-cell activation. Immunol Today 1992;13;431–3. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998;16:111–35. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SRM, Carbone FR, Karamalis F, et al. Help for cytotoxic T cell responses is mediated by CD40 signalling. Nature 1998;393:478–80. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberger SP, Toes REM, van der Voort EIH, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 1998;393:480–3. [DOI] [PubMed] [Google Scholar]
- 5.Callard RE, Armitage RJ, Fanslow WC, et al. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today 1993;14:559–64. [DOI] [PubMed] [Google Scholar]
- 6.Durandy A, Hivroz C, Mazerolles F, et al. Abnormal CD40-mediated activation pathway in B lymphocytes from patients with hyper-IgM syndrome and normal CD40 ligand expression. J Immunol 1997;158:2576–84. [PubMed] [Google Scholar]
- 7.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40 deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1994;1:167–78. [DOI] [PubMed] [Google Scholar]
- 8.Renshaw BR, Fanslow WC, Armitage RJ, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med 1994;180:1889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faris M, Gaskin F, Parsons JT, et al. CD40 signaling pathway: anti-CD40 monoclonal antibody induces rapid dephosphorylation and phosphorylation of tyrosine-phosphorylated proteins including protein tyrosine kinase Lyn, Fyn, and Syk and the appearance of a 28-kD tyrosine phosphorylated protein. J Exp Med 1994;179:1923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren CL, Morio T, Fu SM, et al. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase Cg2. J Exp Med 1994;179:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berberich I, Shu G, Siebelt F, et al. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO J 1996;15:92–101. [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland CL, Heath AW, Pelech SL, et al. Differential activation of the ERK, JNK and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol 1996;157:3381–90. [PubMed] [Google Scholar]
- 13.Hsing Y, Hostager BS, Bishop GA. Characterization of CD40 signaling determinants regulating nuclear factor-κB activation in B lymphocytes. J Immunol 1997;159:4898–906. [PubMed] [Google Scholar]
- 14.Craxton A, Shu G, Graves JD, et al. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol 1998;161:3225–36. [PubMed] [Google Scholar]
- 15.Cheng GH, Cleary AM, Ye ZS, et al. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science 1995;267:1494–8. [DOI] [PubMed] [Google Scholar]
- 16.Ishida T, Tojo T, Aoki T, et al. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci U S A 1996;93:9437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida T, Mizushima S, Azuma S, et al. Identification of TRAF6, a novel tumour necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 1996;271:28745–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto N, Kobayashi N, Azuma S, et al. Two differently regulated nuclear factor kB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci U S A 1999;96:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malinin NL, Boldin MP, Kovalenko AV, et al. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 1997;385:540–4. [DOI] [PubMed] [Google Scholar]
- 20.May MJ, Ghosh S. Signal transduction through NF-κB. Immunol Today 1998;19:80–8. [DOI] [PubMed] [Google Scholar]
- 21.Kashiwada M, Shirakata Y, Inoue JI, et al. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signalling along a Ras-independent pathway. J Exp Med 1998;187:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi CS, Kehrl JH. Activation of stress-activated protein kinase/cJun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J Biol Chem 1997;272:32102–7. [DOI] [PubMed] [Google Scholar]
- 23.Nishitoh H, Saitoh M, Mochida Y, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Molecular Cell 1998;2:389–95. [DOI] [PubMed] [Google Scholar]
- 24.Hollenbaugh D, Mischelpetty N, Edwards CP, et al. Expression of functional CD40 by vascular endothelial cells. J Exp Med 1995;182:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruggiero G, Caceres EM, Voordouw A, et al. CD40 expressed on thymic epithelial cells provides costimulation for proliferation but not apoptosis of human thymocytes. J Immunol 1996;156:3737–46. [PubMed] [Google Scholar]
- 26.Young LS, Eliopoulos AG, Gallagher NJ, et al. CD40 and epithelial cells: across the great divide. Immunol Today 1998;19:502–6. [DOI] [PubMed] [Google Scholar]
- 27.Eliopoulos AG, Dawson CW, Mosialos G, et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 1996;13:2243–54. [PubMed] [Google Scholar]
- 28.Denfeld RW, Hollenbaugh D, Fehrenbach A, et al. CD40 is functionally expressed on human keratinocytes. Eur J Immunol 1996;26:2329–34. [DOI] [PubMed] [Google Scholar]
- 29.Peguet Navarro J, Dalbiez Gauthier C, Moulon C, et al. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol 1997;158:144–52. [PubMed] [Google Scholar]
- 30.Gilligan MG, Knox P, Weedon S, et al. Adenoviral delivery of B7.1 (CD80) increases the immunogenicity of human ovarian and cervical carcinoma cells. Gene Ther 1998;5:965–74. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application of proliferation and cytotoxicity assay. J Immunol Methods 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 32.Schuurhuis GJ, Oberink JW, Bontje PM, et al. The PGP specific combination Syto(R)16/PSC833 detects early apoptosis in CD34 positive progenitor cells. Exp Haematol 1997;25:754. [Google Scholar]
- 33.Miura M, Friedlander RM, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a crmA-sensitive cell death pathway. Proc Natl Acad Sci U S A 1995;92:8318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natoli G, Ianni A, Costanzo A, et al. Resistance to Fas-mediated apoptosis in human hepatoma-cells. Oncogene 1995;11:1157–64. [PubMed] [Google Scholar]
- 35.Watson JM, Sensintaffar JL, Berek JS, et al. Constitutive production of interleukin-6 by ovarian-cancer cell lines and by primary ovarian tumor cultures. Cancer Res 1990;50:6959–65. [PubMed] [Google Scholar]
- 36.Lee LF, Schuerer Maly CC, Lofquist AK, et al. Taxol-dependent transcriptional activation of IL-8 expression in a subset of human ovarian cancer. Cancer Res 1996;56:1303–8. [PubMed] [Google Scholar]
- 37.Glezerman M, Mazot M, Maymon E, et al. Tumor necrosis factor-α and interleukin-6 are differently expressed by fresh human cancerous ovarian tissue and primary cell lines. Eur Cytokine Netw 1998;9:171–9. [PubMed] [Google Scholar]
- 38.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med 1996;183:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eliopoulos AG, Davies C, Knox PG, et al. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumour necrosis factor family. Mol Cell Biol 2000;20:5503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banchereau J, Depaoli P, Valle A, et al. Long-term human B-cell lines dependent on interleukin-4 and antibody to CD40. Science 1991;251:70–2. [DOI] [PubMed] [Google Scholar]
- 41.Baker MP, Fanslow WC, Armitage RJ, et al. Prolonged phenotypic, functional, and molecular change in group I Burkitt lymphoma cells on short-term exposure to CD40 ligand. Blood 1998;92:2830–43. [PubMed] [Google Scholar]
- 42.Randall TD, Heath AW, Santos Argumedo L, et al. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity 1998;8:733–42. [DOI] [PubMed] [Google Scholar]
- 43.Henriquez NV, Floettmann E, Salmon M, et al. Differential responses to CD40 ligation among Burkitt lymphoma lines that are uniformly responsive to Epstein-Barr virus latent membrane protein 1. J Immunol 1999;162:3298–307. [PubMed] [Google Scholar]
- 44.Funakoshi S, Longo DL, Beckwith M, et al. Inhibition of B-cell lymphoma growth by CD40 stimulation. Blood 1994;83:2787–94. [PubMed] [Google Scholar]
- 45.Gaspari AA, Sempowski GD, Chess P, et al. Human epidermal keratinocytes are induced to secrete interleukin 6 and co-stimulate T-lymphocytes. Eur J Immunol 1996;26:1371–7. [DOI] [PubMed] [Google Scholar]
- 46.Eliopoulos AG, Stack M, Dawson CW, et al. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene 1997;14:2899–16. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida S, Ono M, Shono T, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor α-dependent angiogenesis. Mol Cell Biol 1997;17:4015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite–enterocyte contact. Gastroenterology 1997;112:1536–47. [DOI] [PubMed] [Google Scholar]
- 49.Sharma SA, Tummuru MKR, Blaser MJ, et al. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcriptional factor nuclear factor κB in gastric epithelial cells. J Immunol 1998;160:2401–7. [PubMed] [Google Scholar]
- 50.Rothe M, Sarma V, Dixit VM, et al. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 1995;269:1424–7. [DOI] [PubMed] [Google Scholar]
- 51.Traenckner EBM, Pahl HL, Henkel T, et al. Phosphorylation of human IκBα on serine-32 and serine-36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J 1995;14:2876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu L, Kwak YT, Bex F, et al. DNA-dependent protein kinase phosphorylation of IκBα and IκBβ regulates NF-κB DNA binding properties. Mol Cell Biol 1998;18:4221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zandi E, Rothwarf DM, Delhase M, et al. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 1997;91:243–52. [DOI] [PubMed] [Google Scholar]
- 54.May MJ, Ghosh S. Signal transduction—IκB kinases: kinsmen with different crafts. Science 1999;284:271–3. [DOI] [PubMed] [Google Scholar]
- 55.Wood KM, Roff M, Hay RT. Defective IκBα in Hodgkin cell lines with constitutively active NF-κB. Oncogene 1998;16:2131–9. [DOI] [PubMed] [Google Scholar]
- 56.Wu GD, Lai EJ, Huang N, et al. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin 8 promoter. J Biol Chem 1997;272:2396–403. [PubMed] [Google Scholar]
- 57.Eliopoulos AG, Gallagher NJ, Blake SMS, et al. Activation of the p38 MAPK pathway by Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) co-regulates interleukin-6 and interleukin-8 production. J Biol Chem 1999;274:16085–96. [DOI] [PubMed] [Google Scholar]
- 58.Potapova O, Haghighi A, Bost F, et al. The jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitises tumor cells to cisplatin. J Biol Chem 1997;272:14041–4. [DOI] [PubMed] [Google Scholar]
- 59.Lee LF, Li GX, Templeton DJ, et al. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-jun NH2-terminal kinase cJNK (SAPK). J Biol Chem 1998;273:28253–60. [DOI] [PubMed] [Google Scholar]
- 60.Wang TH, Popp DM, Wang HS, et al. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J Biol Chem 1999;274:8208–16. [DOI] [PubMed] [Google Scholar]
- 61.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 1999;93:2999–3007. [PubMed] [Google Scholar]
- 62.Kikuchi T, Crystal RG. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum Gene Ther 1999;10:1375–87. [DOI] [PubMed] [Google Scholar]