Abstract
Chaperonins are oligomeric proteins that assist in the folding of nascent or denatured proteins. Bacterial chaperonins are strongly immunogenic and can cause tissue pathology. They have been implicated in infection, autoimmune disease, and idiopathic or multifactorial diseases, such as arthritis and atherosclerosis. Chaperonin 60 proteins are also involved in prion diseases. In the past few years, much progress has been made in unravelling the involvement of various bacterial and mammalian chaperonin 60 (Cpn 60 or hsp 60) proteins in such diseases, and in proposing mechanisms for their biological actions, although we are still some way from a full understanding of chaperonin action that might lead to immunotherapeutic approaches. This review focuses on the current knowledge of the roles of Cpn 60 in the pathology of infectious and immune diseases, and discusses models for the actions of this molecule. Some potential therapeutic strategies will also be reviewed.
Keywords: Cpn 60, heat shock protein, immunogen
Chaperonins are a subset of the ubiquitous group of proteins known as molecular chaperones.1,2 The chaperonins assist in the correct folding of most proteins in the cell under both normal and stress conditions.3–5 Group I chaperonins (Cpn 60) are prokaryotic proteins, also found in the mitochondria of eukaryotes. Eukaryotes also possess a cytosolic chaperonin known as CCT (chaperonin containing TCP-1), a group II chaperonin. It is not known whether CCT plays a role in disease; therefore, this review is concerned only with the group I chaperonin, Cpn 60.
Chaperonin assisted folding is achieved by sequestration of the misfolded protein in a hydrophobic environment, away from other proteins, to prevent aggregation.6 Chaperonins are essential proteins and are found in almost all prokaryotes and eukaryotes. There are few exceptions: to date, the only organisms shown to lack chaperonins are some parasites, such as microsporidia7 and mycoplasma,8 which have extremely small genomes.
There are two chaperonin proteins, Cpn 60 and Cpn 10, which work together in an oligomeric assembly to achieve the correct folding of proteins, without being involved in the final protein structure themselves.9 However, before their role as protein folding molecules was discovered the chaperonins of certain pathogenic bacteria were identified as major immunogens. In recent years, further evidence of the involvement of bacterial chaperonin proteins in many different aspects of infection and immunity has come to light. Mammalian Cpn 60 proteins are also implicated as endogenous stress signals, and are common suspects in current models of autoimmune disease. These aspects of the chaperonins have taken centre stage and are the focus of intensive research efforts in the fields of bacterial infection, idiopathic diseases such as arthritis and atherosclerosis, and autoimmune conditions. Thus, the chaperonins have emerged not only as crucial in the normal functions of the cell, but as having a central role in mammalian defences against pathogenic attack and the response to damage or stress.
“Chaperonins are essential proteins and are found in almost all prokaryotes and eukaryotes”
In protein folding, Cpn 60 and Cpn 10 work together as parts of the same assembly. However, Cpn 10, being a structurally and functionally different protein,10,11 should perhaps more accurately be described as a “co-chaperone”. Both Cpn 60 and Cpn 10 have roles in pathogenesis and immunity; however, Cpn 10 will not be discussed in this review. For a recent review of the involvement of this protein in infection and immunity see Ranford et al.12
DEFICIENCY OF CPN 60
Cpn 60 is essential for cell survival and, therefore, there are few instances of disease caused by lack of this protein because these are usually fatal. However, there is a temperature sensitive yeast mutant that has mutations in its cpn60 gene, which result in a single amino acid substitution in the Cpn 60 protein. This mutant displays defects in respiration and in steady state mRNA accumulation that can be alleviated by the addition of wild-type Cpn 60.13 There have also been a few reports of Cpn 60 deficiency in humans. Huckriede and Agsteribbe14 reported a patient with systemic mitochondrial encephalopathy, which was caused by multiple deficiencies in mitochondrial enzymes. This patient was found to have lower than normal concentrations of Cpn 60, and this was hypothesised to be the cause of the disease. Another Cpn 60 deficient patient presented with congenital lactic acidaemia, again caused by insufficiency of the mitochondrial enzymes involved in respiration.15 Neither patient was very long lived, thus demonstrating the crucial requirement for Cpn 60.
IMMUNE SYSTEM STIMULATION BY CPN 60
Bacterial Cpn 60 is an important factor in the immune response to many pathogens; in fact, before the identification of “hsp 60” (as it was previously called) as the protein folding homologue of GroEL (the Escherichia coli Cpn 60 and the best studied chaperonin), it was known as “common antigen”. It affects both the innate and acquired immune systems, producing antibody and T cell responses, in addition to innate immune responses. As an example, one fifth of reactive T cells in mice infected with Mycobacterium tuberculosis recognise M tuberculosis Cpn 60.2 (previously known as hsp 65).16 Children immunised with the trivalent diphtheria/pertussis/tetanus vaccine are also found to carry high titres of anti-Cpn 60 antibody,17 and these authors suggest that priming of the immune system to Cpn 60 is a common phenomenon that occurs very early in life.
On the innate immune system front, Cpn 60 proteins from E coli,18,19 M tuberculosis,20 and Chlamydia trachomatis21 all induce the production of pro-inflammatory cytokines by monocytes. Escherichia coli Cpn 60 also directly induces human umbilical vein endothelial cells to produce the adhesion molecules intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin, independently of the normal interleukin 1 (IL-1)/tumour necrosis factor α mediated route of induction.18 These same adhesion molecules were also produced by human vascular epithelium in response to C trachomatis Cpn 60 in a study of atherogenesis.21 In this study, smooth muscle cells and macrophages also reacted to C trachomatis Cpn 60 by producing cytokines. Bacterial Cpn 60 molecules can also induce fibroblasts22 and epithelial cells23 to produce cytokines.
The obvious mechanism by which Cpn 60 might be expected to signal intercellularly is for bacterial or host Cpn 60 to be secreted (or otherwise present outside of the cell), and for a receptor to be present on the surface of certain eukaryotic (host) cell types. There is evidence for both, although reports of extracellular Cpn 60 are controversial. Endogenous Cpn 60 is reported to be expressed on the surface of infected cells,24 but the mechanism for this transport is unknown. Cpn 60 is a mitochondrial protein, possessing a mitochondrial targeting sequence, and there is as yet no known mechanism by which this protein is exported from the cell. It is possible that extracellular Cpn 60 is not the intact oligomeric chaperonin, but a degraded product released from necrotic or damaged cells.
In vitro, at least, it is clear that exogenous Cpn 60 can bind to cells via specific receptors and cause changes in those cells. There are now several reports of receptors for Cpn 60, including the monocyte specific lipopolysaccharide receptor, CD14,25 and the Toll-like receptors (TLRs).26 Furthermore, it was recently reported by Vabulas et al that both human and chlamydial Cpn 60 proteins activate the Toll–IL-1 receptor signalling pathway by binding TLR2 and TLR4. These authors also reported that this activation is dependent on endocytosis of Cpn 60.27
“In vitro, at least, it is clear that exogenous Cpn 60 can bind to cells via specific receptors and cause changes in those cells”
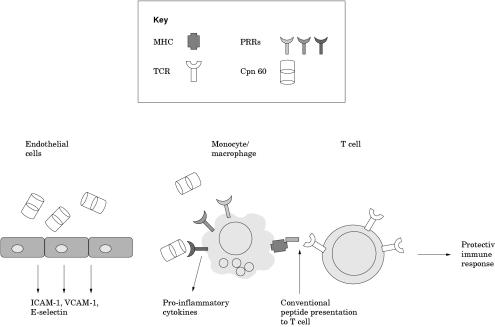
It appears then, that there are multiple receptors for Cpn 60. It is probable that heterogeneity of receptor expression and affinity leads to diversity of response, and that this response is integrated with information about other aspects of the environment, such as the presence of other bacterial components or stress signals. Figure 1 ▶ shows a suggested model of bacterial Cpn 60 induction of the immune system. Clearly though, this is a controversial area in which more work needs to be carried out, so that the extracellular role of Cpn 60 can be more accurately defined.
Figure 1.
Effects of bacterial chaperonin 60 (Cpn 60) on eukaryotic cells. Bacterial Cpn 60 induces vascular endothelial cells to secrete intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin. These play a role in attracting macrophages and lymphocytes to the site of infection. Cpn 60 binds directly to macrophages via pathogen recognition receptors (PPRs), such as the Toll-like receptors, and induces the production and secretion of pro-inflammatory cytokines, thus further enhancing cellular migration to the site of infection. Antigen presenting cells can also present Cpn 60 peptides to T cells on the major histocompatibility complex (MHC), thus inducing the proliferation of T and B cells and initiating a protective immune response. TCR, T cell receptor.
INVOLVEMENT OF CPN 60 IN BONE RESORPTION
In 1995 it was discovered that Cpn 60 from the oral pathogen Actinobacillus actinomycetemcomitans (which is implicated in the pathology of localised juvenile periodontitis, a condition that involves destruction of alveolar bone) is a potent mediator of bone resorption in an in vitro murine calvarial bone model.28 Interestingly, although E coli Cpn 60 was also active in this model, other bacterial Cpn 60 molecules from M tuberculosis and M leprae were not. Further work revealed that E coli Cpn 60 induces the formation of osteoclasts, the multinucleate myeloid bone resorbing cells.29
It is unclear why Cpn 60 molecules from some bacteria stimulate bone resorption whereas others have little or no effect. Differences have also been found in cytokine production by cells that were induced by different Cpn 60s.30 Thus, it is probable that, despite their apparent high homology, there is sufficient variation in the sequence and/or the structure of these homologous chaperonins to result in differences in biological effects.
DIVERGENCE IN CHAPERONIN SEQUENCES AND EFFECTS
The group I chaperonins show extensive sequence similarity with one another; typically around 70%. However, these proteins are not homologous in terms of their effects on cells; a more detailed analysis reveals some important differences. Interestingly, some bacteria, including M tuberculosis, have more than one Cpn 60 protein. During infection, these proteins may have different effects on host cells. For instance, M tuberculosis Cpn 60.1 is a more potent stimulator of monocytes than Cpn 60.2.30 These two proteins share 76% similarity at the amino acid level,31 with most of the divergence being at the C-terminus. Thus, it could be that certain residues at the C-terminus, or even individual residues elsewhere within the protein, account for these differences. Even small changes in amino acid sequence can lead to vast alterations in the function of Cpn 60. For example, in a report by Yoshida et al, a single amino acid substitution in E coli Cpn 60 conferred insecticidal toxicity on this protein.32 Thus, even single amino acid changes can result in large differences in the biological effects of these proteins. This is certainly an aspect of chaperonin research that needs to be investigated because it may provide important clues that could account for the observed differences in immunogenicity between the different proteins.
INVOLVEMENT OF CPN 60 IN AUTOIMMUNE DISEASE
It is still not clear whether host immune responses to exogenous chaperonins are protective or damaging, or indeed, both. It is hypothesised that, given the strong sequence conservation of bacterial and human Cpn 60 (for example, E coli and human Cpn 60 protein sequences share 52% identity and 67% similarity at the amino acid level), T cell mediated immune responses mounted by the host to bacterial Cpn 60 may result in crossreactivity to the host Cpn 60, causing an autoimmune reaction.33
There is increasing evidence of a possible link between bacterial infection and the induction of some autoimmune diseases. For example, self Cpn 60 reactive CD8 T cells were found to cause chronic intestinal inflammatory reactions in mice.34 There is also a convincing case for autoimmunological involvement of Cpn 60 in atherosclerosis.35 Healthy rabbits immunised with M tuberculosis Cpn 60.2 (hsp 65) developed the first inflammatory stage of atherosclerotic plaque formation. Experimentally induced immunosuppression of rabbits blocked the development of Cpn 60.2 induced atherosclerosis, indicating that plaque development entails an immune reaction.36 In human subjects with atherosclerotic lesions in their carotid arteries, the titre of M tuberculosis Cpn 60.2 antibodies was significantly higher than in people without lesions, and these antibodies were shown to crossreact with human Cpn 60.37 Thus, an autoimmune reaction caused by the crossreactivity of antibodies to bacterial Cpn 60 with the human homologue is one factor in what is a multifactorial disease, with the involvement of classic risk factors such as a high cholesterol diet and the lack of apolipoprotein E also playing their part.38
Autoimmunity to Cpn 60 may also be a factor in infertility and fetal/embryo loss. Chlamydial infections are a common cause of infertility, owing to damage or occlusion of the fallopian tubes. This damage might be caused by antichlamydial antibodies that are crossreactive with human Cpn 60.39 It was found that women undergoing in vitro fertilisation who had IgA in their cervical mucosa to Cpn 60 of C trachomatis, as a result of a previous or ongoing chlamydial infection, were less likely to become pregnant.40 In another experiment, fertilised mouse embryos that were incubated in maternal sera containing antibodies reactive to mouse Cpn 60 developed less well than those not exposed to such antibodies.41 The ability of human Cpn 60 to elicit a cell mediated immune response also correlates with early stage pregnancy loss.42 In a clinical evaluation of factors involved in adverse pregnancy outcome, Cpn 60–antibody complexes were found in the placenta of a high proportion of preterm births.43 These findings suggest that the crossreactivity of antibodies to human Cpn 60 may be a cause of many difficulties encountered in conception, pregnancy, and birth.
Paradoxically, however, it seems that in some situations Cpn 60 may inhibit or ameliorate the progression of some autoimmune diseases. In a murine model of insulin dependent diabetes mellitus, a Cpn 60 peptide was identified as a target for the T cell clones that were attacking the pancreatic islets. However, the administration of this peptide early in life appeared to prevent the onset of this disease.44,45 Administration of Cpn 60 in a rat model of adjuvant arthritis also produces a protective effect, probably through modulation of T cell function.46
PRION DISEASES
Prions are transmissible elements that are thought to be composed only of protein.47 The prion protein, PrP, is a host encoded glycoprotein that can exist in an abnormal, pathogenic isoform known as PrPSc.48 PrPSc is thought to be the infectious agent. It is hypothesised that PrPSc acts as a template upon which normal cellular PrP, which contains almost no β sheet structure, is induced to convert to the β sheet containing PrPSc isoform, thus forming fibrillar aggregates that cause disease.49 Little is known about the mechanism of conversion of the normal PrP protein to the PrPSc isotype. However, human Cpn 60, presumably in its “protein folding” role, has been shown to interact specifically with PrP in a yeast two hybrid screen.50 More recently, it has been shown that E coli Cpn 60 actively promotes conversion to the PrPSc form in vitro.51 It is important to note that these results are from in vitro experiments, and there is as yet no evidence that PrP and Cpn 60 can interact in vivo, because they are not known to occur in the same cellular compartments. However, this is clearly a very exciting area of research that has the potential to yield greater understanding of the spongiform encephalopathies and possible therapeutic intervention.
Take home messages.
Chaperonins are oligomeric proteins that assist in the folding of nascent or denatured proteins
Bacterial chaperonins are strongly immunogenic and can cause tissue pathology, being implicated in infection, autoimmune disease, prion diseases, and idiopathic or multifactorial diseases, such as arthritis and atherosclerosis
Recently, much progress has been made in elucidating the roles of various bacterial and mammalian chaperonin 60 (Cpn 60 or hsp 60) proteins in these diseases and determining their mechanism of action
Exogenous and/or endogenous Cpn 60 is found in the external milieu of cells and can activate certain cell types via one or more receptors
More research into this area is needed because a more complete understanding of chaperonin action might lead to immunotherapeutic approaches to many life threatening diseases
MODELS OF CPN 60 ACTION
The current dogma is that the immune system evolved to distinguish between “self” and “non-self”. However, the basic self–non-self model fails to explain some aspects of immunity, and it has been modified by Janeway52,53 to include molecules recognised by cells of the innate immune system, in addition to foreign molecules recognised by T and B cells. The molecules that might be recognised by innate immune cells such as macrophages and dendritic cells are common to many bacteria, parasites, and fungi, and could be generically recognised. Such molecules have been termed pathogen associated molecular patterns (PAMPs), and might include—for example, lipopolysaccharide of Gram negative bacteria, mannans such as lipoarabinomannan from mycobacteria, or viral double stranded RNA. Given their ubiquitous nature and high interspecies homology, and because it has now been reported that chaperonin proteins are found extracellularly, these proteins are another possible candidate for PAMPs.
“Cpn 60 is ideally placed to play a central role in the initiation of the host immune response, whatever the actual nature of the immune system”
The idea of chaperonins as initiators of immune responses also fits in increasingly well, but in a different way, with another model of the immune system: the “danger” model.54 This idea proposes that, instead of the major function of the immune system being to discriminate between self and non-self, its function is actually to recognise “danger”; specifically, certain “danger signals” that emanate from the tissues of the body, rather than from pathogens. Thus, molecules released from stressed, damaged, and necrotic host cells would be seen by the immune system as signals of infection or wounding, and the appropriate response would be mounted. In this model, it is self Cpn 60 that is being recognised, not the Cpn 60 of the pathogen. Heat shock proteins such as Cpn 60 might be a good example of a danger signal, because they are themselves induced by stress or damage and would be present in large amounts in tissue containing stressed, damaged, or lysed cells. Thus, it would seem that Cpn 60 is ideally placed to play a central role in the initiation of the host immune response, whatever the actual nature of the immune system.
THERAPEUTIC POSSIBILITIES
There is still much to be learned about the roles of chaperonins in pathology and the immune system and, given their diverse effects, at this stage it might seem rather premature to consider their use in prophylaxis or treatment. However, as detailed earlier, there are already examples of the protective effect of immunisation with Cpn 60 or specific Cpn 60 peptides in animal models of autoimmune disease.44,55 The crossreactivity with human Cpn 60, seen in many autoimmune diseases, suggests that early tolerisation to the protein by immunisation might protect against the development of pathology.
The inflammatory effects of the chaperonins caused by their cytokine stimulatory action could theoretically be ameliorated by the use of anti-Cpn 60 antibodies, particularly antibodies that do not crossreact with human Cpn 60. However, the problem with all these potential treatments is that Cpn 60 seems to have diverse and complex functions in the immune system, and this might lead to unforeseen effects of treatment. The task ahead is to identify the benefits and risks of such intervention. There is greater potential, at the present time, for Cpn 60 related treatments to specific diseases, rather than a cure all approach. Doubtless, as our knowledge and understanding of the role of Cpn 60 in infection and immunity grows, we will be more able to prevent and treat many currently disabling and life threatening diseases, and to gain more insight into this biologically and medically fascinating molecule.
Acknowledgments
The authors thank S Nair for critical evaluation of the manuscript. We thank the BBSRC, the Arthritis Research Campaign, and the British Heart Foundation for funding.
Abbreviations
CCT, chaperonin containing TCP-1
Cpn, chaperonin
IL-1, interleukin 1
PAMPs, pathogen associated molecular patterns
PrP, prion protein
PrPSc, abnormal, pathogenic isoform of PrP
TLR, Toll-like receptor
REFERENCES
- 1.Morimoto RI, Tissiéres A, Georgopoulos C, eds. The biology of heat shock proteins and molecular chaperones. Plainview, NY: Cold Spring Harbor Laboratory Press, 1994.
- 2.Hartl FU. Molecular chaperones in cellular protein folding. Nature 1996;381:571–9. [DOI] [PubMed] [Google Scholar]
- 3.Hemmingsen SM, Woolford C, van der Vies SM, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature 1988;333:330–4. [DOI] [PubMed] [Google Scholar]
- 4.Fink AL. Chaperone-mediated protein folding. Physiol Rev 1999;79:425–49. [DOI] [PubMed] [Google Scholar]
- 5.Houry WA, Frishman D, Eckerskorn C, et al. Identification of in vivo substrates of the chaperonin GroEL. Nature 1999;402:147–54. [DOI] [PubMed] [Google Scholar]
- 6.Fenton WA, Horwich AL. GroEL-mediated protein folding. Protein Sci 1997;6:743–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katinka MD, Duprat S, Cornillot E, et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 2001;414:450–3. [DOI] [PubMed] [Google Scholar]
- 8.Glass JI, Lefkowitz EJ, Glass, JS, et al. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature 2000;407:757–62. [DOI] [PubMed] [Google Scholar]
- 9.Ranson NA, White HE, Saibil HR. Chaperonins. Biochem J 1998;333:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langer T, Pfeifer G, Martin J, et al. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J 1992;11:4757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin J, Mayhew M, Langer T, et al. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature 1993;366:228–33. [DOI] [PubMed] [Google Scholar]
- 12.Ranford J, Coates A, Henderson B. Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Reviews in Molecular Medicine 2000;15 September (http://www.ermm.cbcu.cam.ac.uk/00002015h.htm). [DOI] [PubMed]
- 13.Sanyal A, Harington A, Herbert CJ, et al. Heat shock protein HSP60 can alleviate the phenotype of mitochondrial RNA-deficient temperature-sensitive mna2 pet mutants. Mol Gen Genet 1995;246:56–64. [DOI] [PubMed] [Google Scholar]
- 14.Huckriede A, Agsteribbe E. Decreased synthesis and inefficient mitochondrial import of hsp60 in a patient with a mitochondrial encephalomyopathy. Biochim Biophys Acta 1994;1227:200–6. [DOI] [PubMed] [Google Scholar]
- 15.Briones P, Vilaseca MA, Ribes A, et al. A new case of multiple mitochondrial enzyme deficiencies with decreased amount of heat shock protein 60. J Inherit Metab Dis 1997;20:569–77. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann SH, Vath U, Thole JE, et al. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol 1987;17:351–7. [DOI] [PubMed] [Google Scholar]
- 17.Del Giudice G, Gervaix A, Costantino P, et al. Priming to heat shock proteins in infants vaccinated against pertussis. J Immunol 1993;150:2025–32. [PubMed] [Google Scholar]
- 18.Galdiero M, de l’Ero GC, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun 1997;65:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabona P, Reddi K, Khan S, et al. Homogeneous Escherichia coli chaperonin 60 induces IL-1β and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J Immunol 1998;161:1414–21. [PubMed] [Google Scholar]
- 20.Friedland JS, Shattock R, Remick DG, et al. Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin Exp Immunol 1993;91:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kol A, Bourcier T, Lichtman AH, et al. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 1999;103:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinode D, Yoshioka M, Tanabe S, et al. The GroEL-like protein from Campylobacter rectus: immunological characterization and interleukin-6 and -8 induction in human gingival fibroblast. FEMS Microbiol Lett 1998;167:1–6. [DOI] [PubMed] [Google Scholar]
- 23.Marcatili A, Cipollaro de l’Ero G, Galdiero M, et al. TNF-alpha, IL-1 alpha, IL-6 and ICAM-1 expression in human keratinocytes stimulated in vitro with Escherichia coli heat-shock proteins. Microbiology 1997;143:45–53. [DOI] [PubMed] [Google Scholar]
- 24.Belles C, Kuhl A, Nosheny R, et al. Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect Immun 1999;67:4191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kol A, Lichtman AH, Finberg RW, et al. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol 2000;164:13–17. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi K, Burkart V, Flohe S, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 2000;164:558–61. [DOI] [PubMed] [Google Scholar]
- 27.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 2001;276:31332–9. [DOI] [PubMed] [Google Scholar]
- 28.Kirby AC, Meghji S, Nair SP, et al. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone GroEL. J Clin Invest 1995;96:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddi K, Meghji S, Nair SP, et al. The Escherichia coli chaperonin 60 (GroEL) is a potent stimulator of osteoclast formation. J Bone Miner Res 1998;13:1260–6. [DOI] [PubMed] [Google Scholar]
- 30.Lewthwaite JC, Coates ARM, Tormay P, et al. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect Immun 2001;69:7349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong TH, Coates ARM, Butcher PD, et al. Mycobacterium tuberculosis expresses two chaperonin-60 homologs. Proc Natl Acad Sci U S A 1993;90:2608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida N, Oeda K, Watanabe E, et al. Protein function. Chaperonin turned insect toxin. Nature 2001;411:44. [DOI] [PubMed] [Google Scholar]
- 33.van Eden W, van der Zee R, Taams LS, et al. Heat-shock protein T-cell epitopes trigger a spreading regulatory control in a diversified arthritogenic T-cell response. Immunol Rev 1998;164:169–74. [DOI] [PubMed] [Google Scholar]
- 34.Steinhoff U, Brinkmann V, Klemm U, et al. Autoimmune intestinal pathology induced by hsp60-specific CD8 T cells. Immunity 1999;11:349–58. [DOI] [PubMed] [Google Scholar]
- 35.Wick G, Perschinka H, Xu Q. Autoimmunity and atherosclerosis. Am Heart J 1999;138:S444–9. [DOI] [PubMed] [Google Scholar]
- 36.Metzler B, Mayr M, Dietrich H, et al. Inhibition of arteriosclerosis by T-cell depletion in normocholesterolemic rabbits immunized with heat shock protein 65. Arterioscler Thromb Vasc Biol 1999;19:1905–11. [DOI] [PubMed] [Google Scholar]
- 37.Mayr M, Metzler B, Kiechl S, et al. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 1999;99:1560–6. [DOI] [PubMed] [Google Scholar]
- 38.Wick G. Atherosclerosis—an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz 2000;25:87–90. [DOI] [PubMed] [Google Scholar]
- 39.Neuer A, Spandorfer SD, Giraldo P, et al. The role of heat shock proteins in reproduction. Hum Reprod Update 2000;6:149–59. [DOI] [PubMed] [Google Scholar]
- 40.Witkin SS, Sultan KM, Neal GS, et al. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol 1994;171:1208–14. [DOI] [PubMed] [Google Scholar]
- 41.Neuer A, Mele C, Liu HC, et al. Monoclonal antibodies to mammalian heat shock proteins impair mouse embryo development in vitro. Hum Reprod 1998;13:987–90. [DOI] [PubMed] [Google Scholar]
- 42.Witkin SS. Immunity to heat shock proteins and pregnancy outcome. Infect Dis Obstet Gynecol 1999;7:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziegert M, Witkin SS, Sziller I, et al. Heat shock proteins and heat shock protein–antibody complexes in placental tissues. Infect Dis Obstet Gynecol 1999;7:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias D, Markovits D, Reshef T, et al. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A 1990;87:1576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elias D, Cohen IR. The hsp60 peptide p277 arrests the autoimmune diabetes induced by the toxin streptozotocin. Diabetes 1996;45:1168–72. [DOI] [PubMed] [Google Scholar]
- 46.van Eden W, Wendling U, Paul L, et al. Arthritis protective regulatory potential of self-heat shock protein cross-reactive T cells. Cell Stress Chaperones 2000;5:452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffith JS. Self-replication and scrapie. Nature 1967;215:1043–4. [DOI] [PubMed] [Google Scholar]
- 48.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982;216:136–44. [DOI] [PubMed] [Google Scholar]
- 49.Pan KM, Baldwin M, Nguyen J, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 1993;90:10962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edenhofer F, Rieger R, Famulok M, et al. Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J Virol 1996;70:4724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DebBurman SK, Raymond GJ, Caughey B, et al. Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc Natl Acad Sci U S A 1997;94:13938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 1992;13:11–16. [DOI] [PubMed] [Google Scholar]
- 53.Janeway CA, Jr. How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci U S A 2001;98:7461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 55.van Eden W, Hogervorst EJ, van der Zee R, et al. The mycobacterial 65 kD heat-shock protein and autoimmune arthritis. Rheumatol Int 1989;9:187–91. [DOI] [PubMed] [Google Scholar]