Abstract
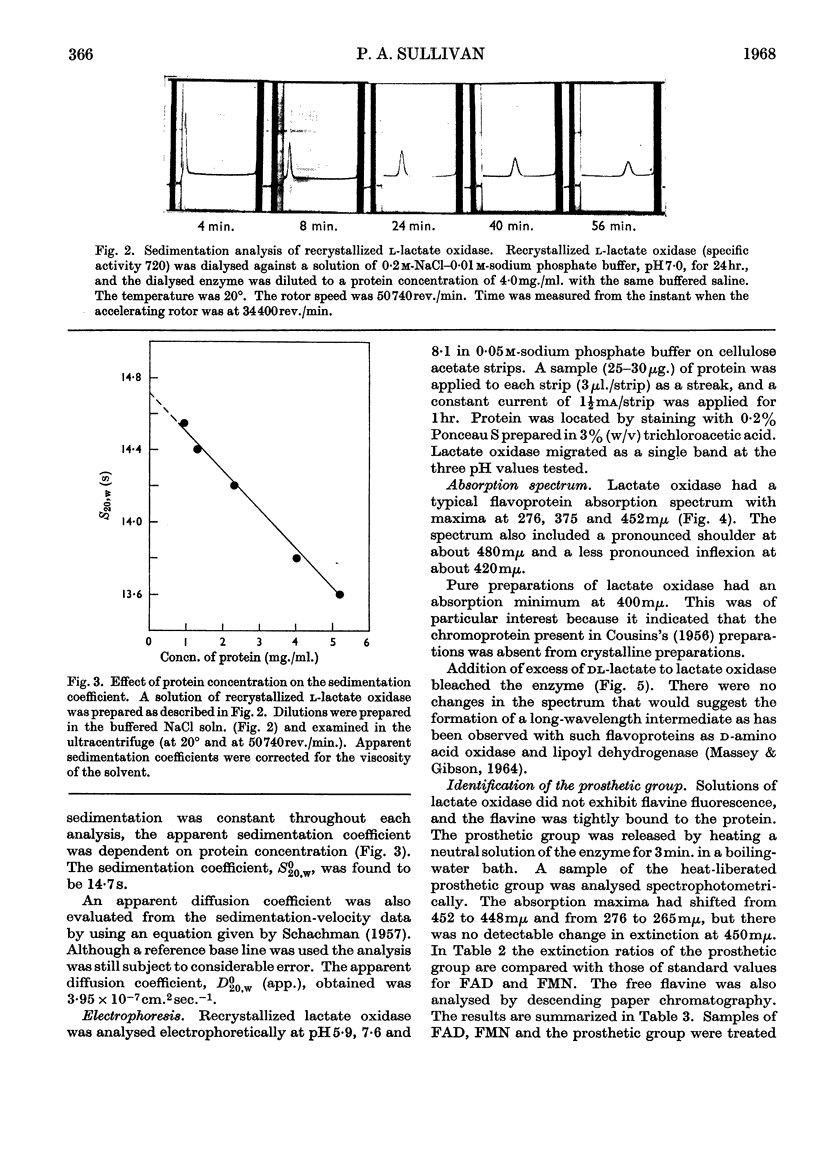
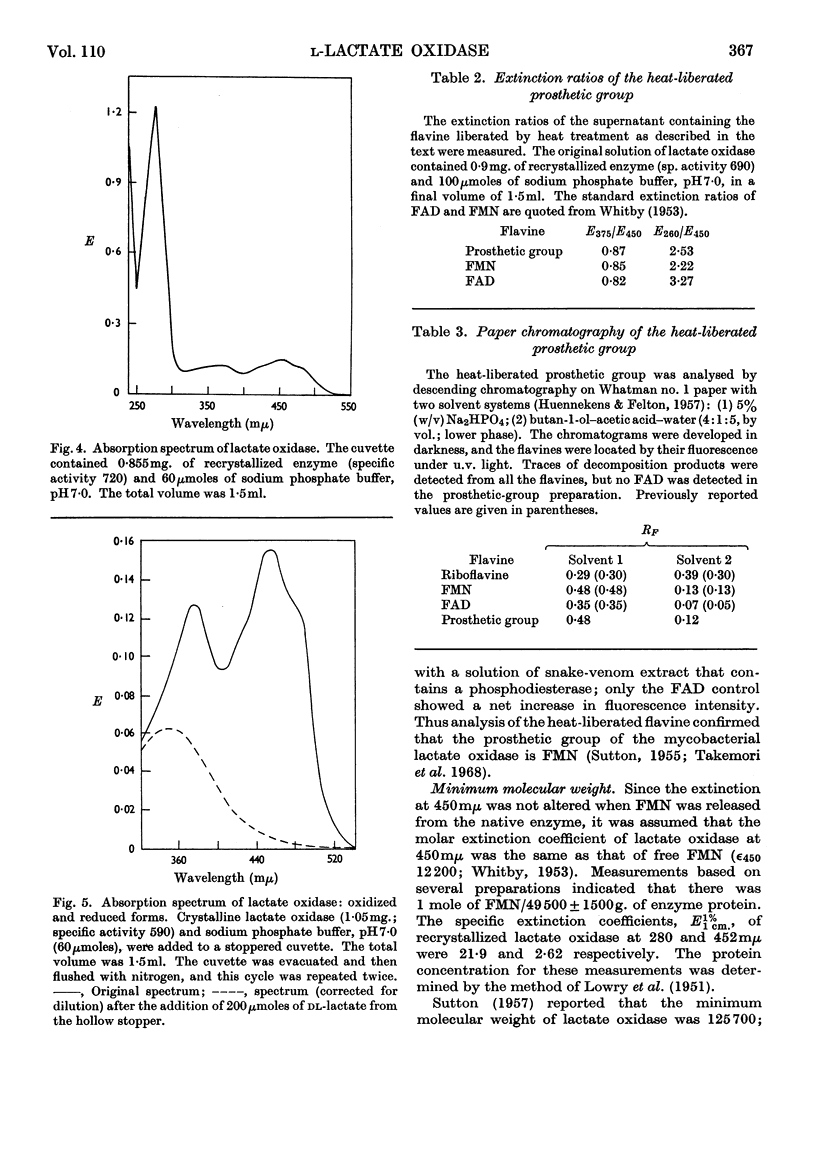
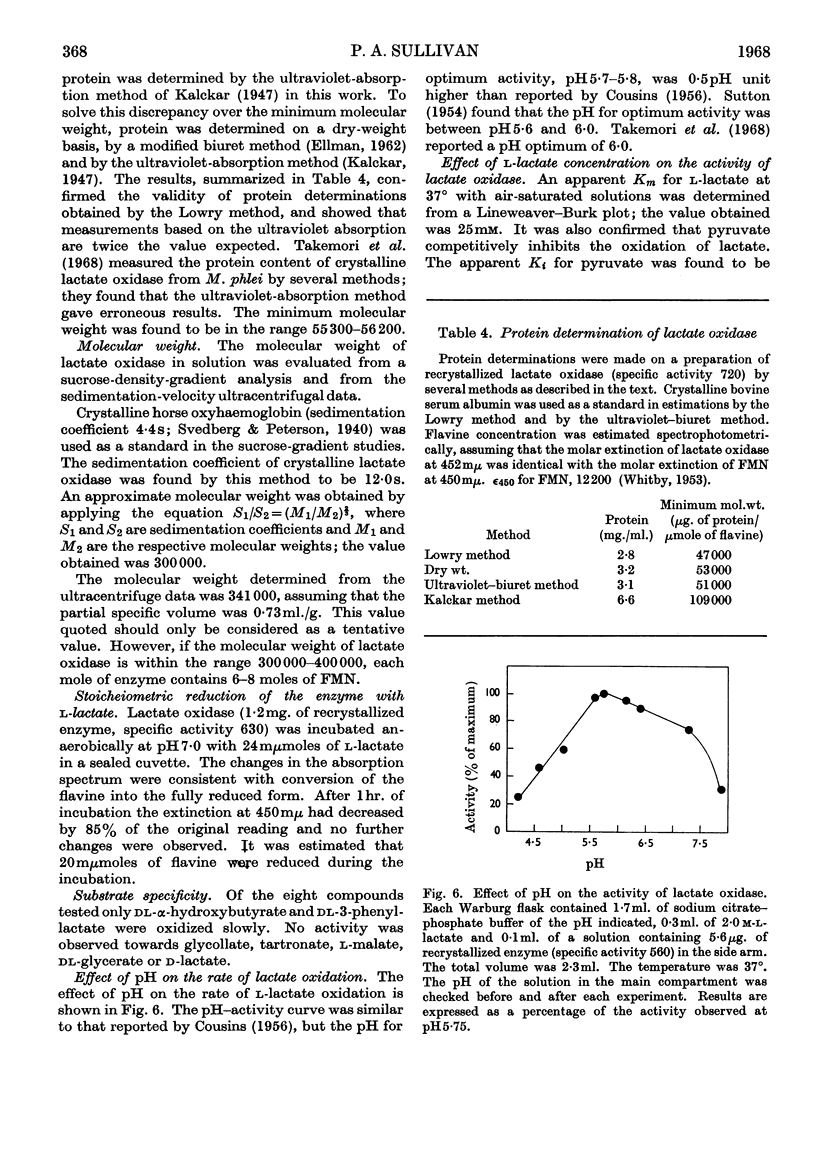
1. An original method was devised for purifying and crystallizing l-lactate oxidase from Mycobacterium smegmatis. 2. The crystalline enzyme exhibited a single protein component (S020,w 14·7s) in the ultracentrifuge and also on electrophoresis on cellulose acetate strips. 3. The enzyme has a typical flavoprotein spectrum, and it was confirmed that the prosthetic group is FMN. 4. Preliminary studies indicated that the molecular weight is in the range 300000–400000. Since the minimum molecular weight was found to be 50000±3000, it was concluded that l-lactate oxidase contains 6–8moles of flavine/mole. 5. Other properties of the enzyme reported include the substrate specificity, an apparent Km for l-lactate, the effect of several inhibitors on the enzyme activity and the pH–activity curve.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COUSINS F. B. The lactic acid oxidase of the mycobacteria. Biochem J. 1956 Oct;64(2):297–304. doi: 10.1042/bj0640297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. The biuret reaction: changes in the ultraviolet absorption spectra and its application to the determination of peptide bonds. Anal Biochem. 1962 Jan;3:40–48. doi: 10.1016/0003-2697(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Edson N. L. The oxidation of lactic acid by Mycobacterium phlei. Biochem J. 1947;41(2):145–151. doi: 10.1042/bj0410145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichel H. J. Oxidation of L-alpha-hydroxy acids by Tetrahymena pyriformis. Biochim Biophys Acta. 1966 Oct 17;128(1):183–186. doi: 10.1016/0926-6593(66)90156-1. [DOI] [PubMed] [Google Scholar]
- KOHN J. A cellulose acetate supporting medium for zone electrophoresis. Clin Chim Acta. 1957 Aug;2(4):297–303. doi: 10.1016/0009-8981(57)90005-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MASSEY V., GIBSON Q. H. ROLE OF SEMIQUINONES IN FLAVOPROTEIN CATALYSIS. Fed Proc. 1964 Jan-Feb;23:18–29. [PubMed] [Google Scholar]
- SUTTON W. B. Isolation and properties of a lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1954 Sep;210(1):309–320. [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- SUTTON W. B. Sulfhydryl and prosthetic groups of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1955 Oct;216(2):749–761. [PubMed] [Google Scholar]
- Takemori S., Nakazawa K., Nakai Y., Suzuki K., Katagiri M. A lactate oxygenase from Mycobacterium phlei. Improved purification and some properties of the enzyme. J Biol Chem. 1968 Jan 25;243(2):313–319. [PubMed] [Google Scholar]
- UDAKA S., KOUKOL J., VENNESLAND B. Lactic oxidase of Pneumococcus. J Bacteriol. 1959 Nov;78:714–725. doi: 10.1128/jb.78.5.714-725.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]




