Abstract
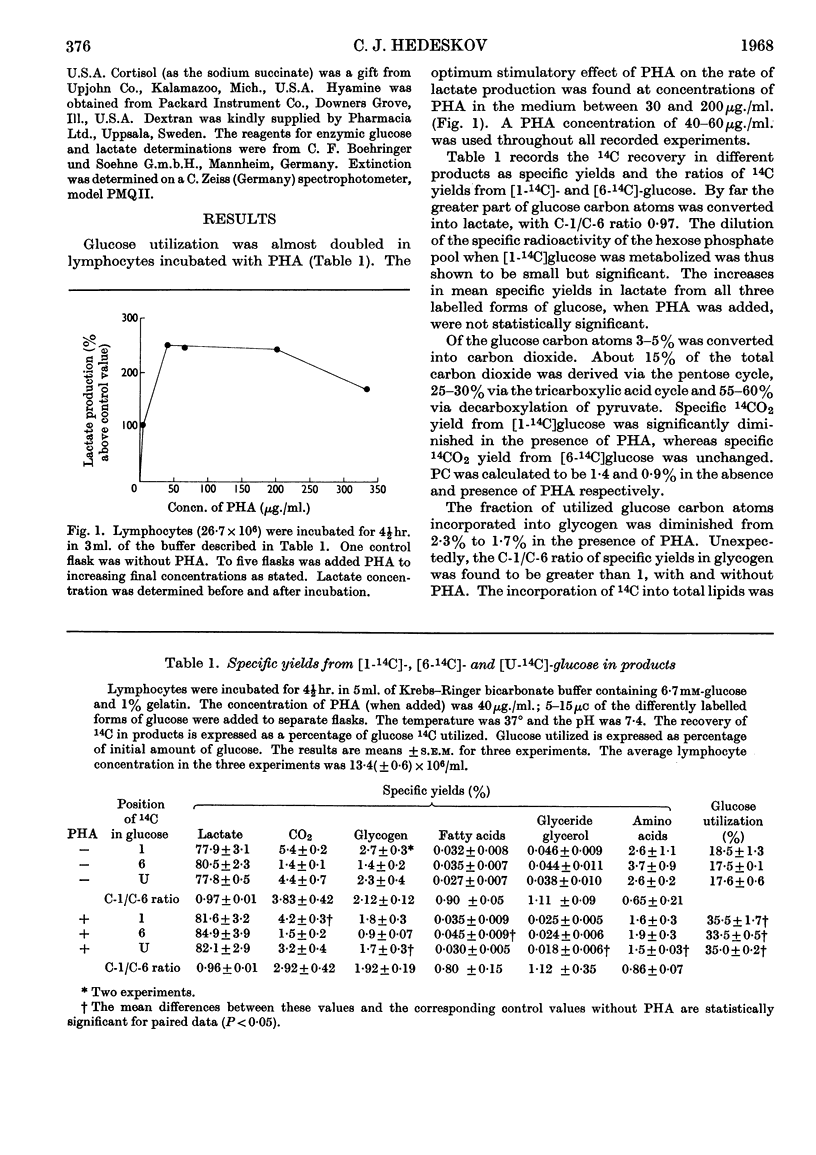
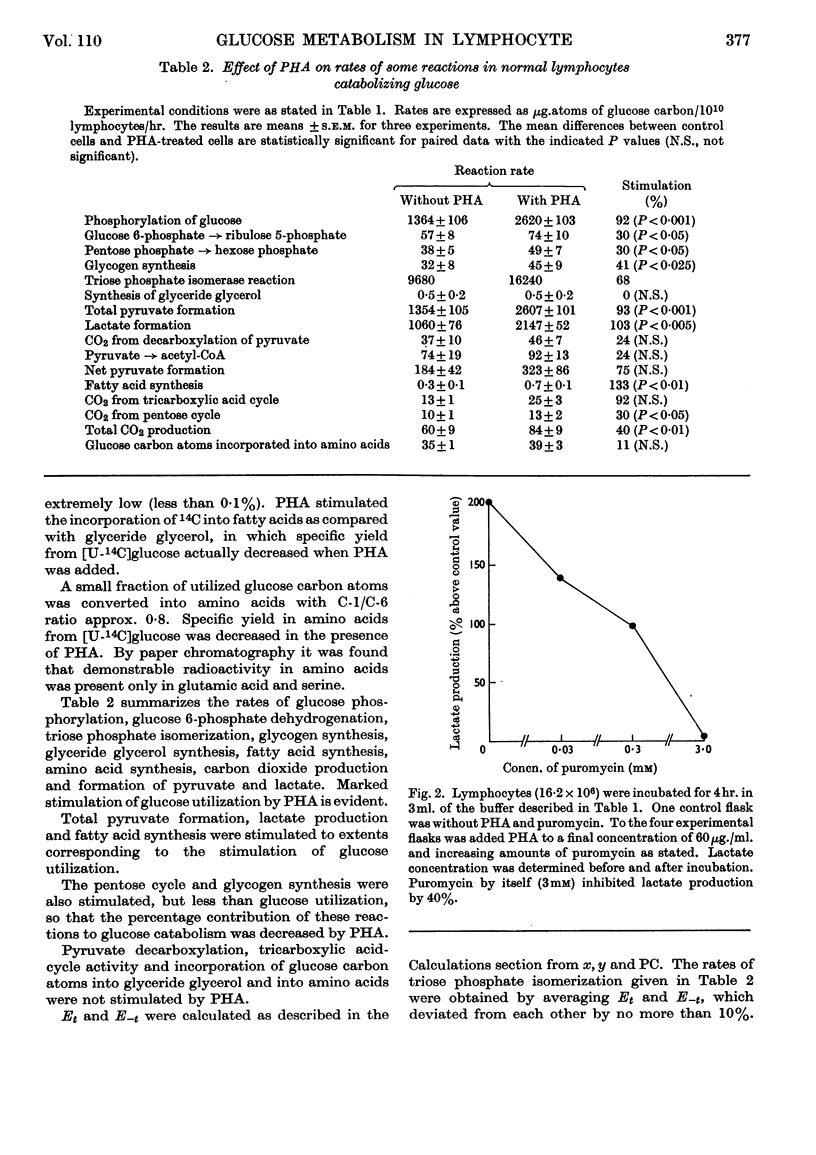
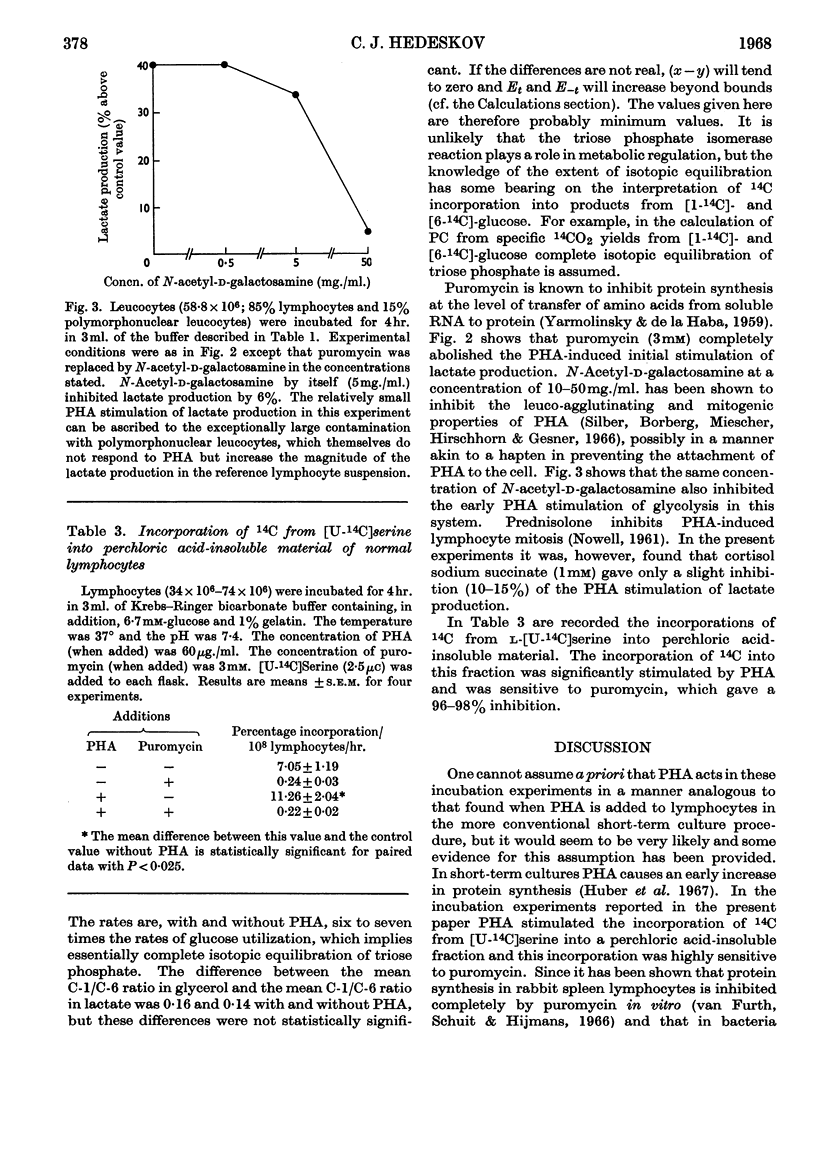
1. Phytohaemagglutinin induced early changes in the catabolism of glucose by normal human lymphocytes suspended in a bicarbonate buffer. During 4hr. incubation glucose utilization was almost doubled. 2. The rates of several reactions in the metabolism of glucose were estimated. Total pyruvate formation, lactate production and fatty acid synthesis were stimulated to the same degree as was glucose utilization. The pentose cycle and the glycogen synthesis were also stimulated but less than was glucose utilization. The pentose cycle was found to account for 1·4% and 0·9% of the total glucose utilization without and with phytohaemagglutinin respectively. In these cells rates of triose phosphate iso-merization were at least six to seven times the rate of glucose phosphorylation. On an average 55–60% of the total carbon dioxide evolved was derived from decarboxylation of pyruvate, 25–30% from the tricarboxylic acid cycle and about 15% from the pentose cycle. Observed ratios of 14C specific yields in glycogen from [1-14C]- and [6-14C]-glucose could possibly be explained by assuming the existence of two separate glucose 6-phosphate pools. 3. During 4hr. incubation in bicarbonate buffer 14C from [U-14C]serine was incorporated into perchloric acid-insoluble material. This incorporation was stimulated by phytohaemagglutinin but was almost completely inhibited by puromycin. Puromycin also abolished phytohaemagglutinin-induced stimulation of glycolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSCH H., HURLBERT R. B., POTTER V. R. Anion exchange chromatography of acids of the citric acid cycle. J Biol Chem. 1952 May;196(2):717–727. [PubMed] [Google Scholar]
- Barker B. E., Farnes P. Histochemistry of blood cells treated with pokeweed mitogen. Nature. 1967 May 20;214(5090):787–789. doi: 10.1038/214787a0. [DOI] [PubMed] [Google Scholar]
- COOPER H. L., RUBIN A. D. RNA METABOLISM IN LYMPHOCYTES STIMULATED BY PHYTOHEMAGGLUTININ: INITIAL RESPONSES TO PHYTOHEMAGGLUTININ. Blood. 1965 Jun;25:1014–1027. [PubMed] [Google Scholar]
- Forsdyke D. R. Quantitiative nucleic acid changes during phytohaemagglutinin-induced lymphocyte transformation in vitro. Dependence of the response on phytohaemagglutinin-serum rati. Biochem J. 1967 Nov;105(2):679–684. doi: 10.1042/bj1050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMREICH E., EISEN H. N. The distribution and utilization of glucose in isolated lymph node cells. J Biol Chem. 1959 Aug;234(8):1958–1965. [PubMed] [Google Scholar]
- Hedeskov C. J., Esmann V. Major metabolic pathways of glucose in normal human lymphocytes and the effect of cortisol. Biochim Biophys Acta. 1967 Nov 28;148(2):372–383. doi: 10.1016/0304-4165(67)90133-x. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J., Esmann V. Respiration and glycolysis of normal human lymphocytes. Blood. 1966 Aug;28(2):163–174. [PubMed] [Google Scholar]
- Hrachovec J. P. The effect of phytohemagglutinin on glucose-U-C14 oxidation by peritoneal and blood white cells of the rat. J Cell Physiol. 1966 Jun;67(3):399–405. doi: 10.1002/jcp.1040670305. [DOI] [PubMed] [Google Scholar]
- Huber H., Winkler H., Reiser G., Huber C., Gabl F., Braunsteiner H. Eigenschaften und subcelluläre Lokalisation neugebildeter Proteine menschlicher Lymphocyten in vitro mit und ohne Phytohämagglutininzusatz. Klin Wochenschr. 1967 Feb 15;45(4):204–210. doi: 10.1007/BF01716909. [DOI] [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- MacHaffie R. A., Wang C. H. The effect of phytohemagglutinin upon glucose catabolism in lymphocytes. Blood. 1967 Apr;29(4 Suppl):640–646. [PubMed] [Google Scholar]
- Mueller G. C., Mahieu M. L. Induction of ribonucleic acid synthesis in human leucocytes by phytohemagglutinin. Biochim Biophys Acta. 1966 Jan 18;114(1):100–107. doi: 10.1016/0005-2787(66)90257-7. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Inhibition of human leukocyte mitosis by prednisolone in vitro. Cancer Res. 1961 Dec;21:1518–1521. [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- PEARMAIN G., LYCETTE R. R., FITZGERALD P. H. Tuberculin-induced mitosis in peripheral blood leucocytes. Lancet. 1963 Mar 23;1(7282):637–638. doi: 10.1016/s0140-6736(63)91275-3. [DOI] [PubMed] [Google Scholar]
- Parenti F., Franceschini P., Forti G., Cepellini R. The effect of phytohaemagglutinin on the metabolism and gamma-globulin synthesis of human lymphocytes. Biochim Biophys Acta. 1966 Jul 20;123(1):181–187. doi: 10.1016/0005-2787(66)90171-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAGLINO D., HAYHOE F. G., FLEMANS R. J. Cytochemical observations on the effect of phytohaemagglutinin in short-term tissue cultures. Nature. 1962 Oct 27;196:338–340. doi: 10.1038/196338a0. [DOI] [PubMed] [Google Scholar]
- SCHREK R., RABINOWITZ Y. Effects of phytohemagglutinin on rat and normal and leukemic human blood cells. Proc Soc Exp Biol Med. 1963 May;113:191–194. doi: 10.3181/00379727-113-28317. [DOI] [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Schuit R. E., Hijmans W. The formation of immunoglobulins by human tissues in vitro. I. The methods and their specificity. Immunology. 1966 Jul;11(1):1–11. [PMC free article] [PubMed] [Google Scholar]


