Abstract
Periodontitis is a multifactorial immune‐mediated disease exacerbated by dysregulated alveolar bone homeostasis. Timely intervention is crucial for disease management to prevent tooth loss. To successfully manage periodontitis, it is imperative to understand the cellular and molecular mechanisms involved in its pathogenesis to develop novel treatment modalities. Non‐surgical periodontal therapy (NSPT) such as subgingival instrumentation/debridement has been the underlying treatment strategy over the past decades. However, new NSPT approaches that target key signaling pathways regulating alveolar bone homeostasis have shown positive clinical outcomes. This narrative review aims to discuss endogenous bone homeostasis mechanisms impaired in periodontitis and highlight the clinical outcomes of preventive periodontal therapy to avoid invasive periodontal therapies. Although the anti‐resorptive therapeutic adjuncts have demonstrated beneficial outcomes, adverse events have been reported. Diverse immunomodulatory therapies targeting the osteoblast/osteoclast (OB/OC) axis have shown promising outcomes in vivo. Future controlled randomized clinical trials (RCT) would help clinicians and patients in the selection of novel preventing therapies targeting key molecules to effectively treat or prevent periodontitis.
Keywords: alveolar bone homeostasis, osteoblasts, osteoclasts, periodontal therapy, periodontitis, RANK/RANKL signaling
Anti‐resorptive, immunomodulatory, and emerging therapeutic approaches to treat periodontal disease and promote resolution of inflammation.

1. INTRODUCTION
Periodontitis is a chronic, osteolytic disease characterized by the loss of supporting tissues of the dentition including the periodontal ligament, cementum, and alveolar bone (the periodontium). 1 , 2 , 3 Epidemiologic studies indicate that periodontitis is one of the most common inflammatory non‐communicable diseases (NCDs) and it is reported to be the sixth most prevalent disease condition in the world with at least 10% of the global adult population suffering from the severe form of periodontitis. 4 , 5 , 6 , 7 , 8 This multifactorial disease is caused by the impaired crosstalk between the host and the microbial biofilm, promoting an environment that favors bone resorption. 9 , 10 , 11 , 12 The well‐known pathogenic components of oral biofilm are the gram‐negative anaerobic species Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa) that alter the resident commensal population important in maintaining gingival tissue homeostasis. 13 , 14 The chronic inflammation due to uncontrolled expression of virulence factors, viz. potent osteoclast stimulators such as Pg‐encoded Arg and Lys‐gingipain cysteine proteinases and Aa‐derived heat shock proteins, accelerates bone resorption and stimulates inflammation by fibroblast‐induced IL6 resulting in an imbalance in osteoblasts (OB; bone‐forming cells) and osteoclasts (OC; bone‐resorbing cells) activity impairing alveolar bone homeostasis. 15 , 16 , 17 , 18 Figure 1 describes the anatomic differences between healthy and inflamed gingiva. It is noteworthy that although periodontitis is generally considered an aging‐associated disease, young populations (≤35 years of age) are also experiencing this chronic disease with greater frequency. 19 , 20 Furthermore, it is well reported that periodontitis is associated with other chronic diseases such as rheumatoid arthritis, neurologic disorders, cardiovascular disease, preterm low birth weight, diabetes, and cancer. 21 , 22 , 23 Thus, new directions of periodontal research and novel treatment strategies are urgently needed. This narrative review aims to provide an update on the current concepts regarding dysregulated cellular and molecular mechanisms associated with the OB/OC imbalance impairing alveolar bone homeostasis in periodontitis and to provide an update of new preventive and regenerative therapeutic approaches.
FIGURE 1.

The anatomic structure of the periodontium in healthy and inflamed gingiva. Healthy gingiva is firmly attached to the tooth surface. The periodontal ligament, cementum, gingiva, and alveolar bone remain intact without impairment in their anatomic structure. However, in inflamed gingiva, periodontal pocket formation is the cardinal sign of periodontitis due to the dysbiosis caused by pathogenic biofilm. Thereafter, an overreactive immune‐inflammatory response is observed by the recruitment of neutrophils in the periodontal pocket along with an infiltration and proliferation of myeloid and lymphocyte cells ultimately leading to clinical manifestations of redness, edema, and bleeding.
2. OVERT IMMUNE RESPONSES TRIGGER PERIODONTAL PATHOPHYSIOLOGY
Alveolar bone homeostasis is regulated by the coupled crosstalk of OB/OC wherein the rate of bone formation and resorption are in equilibrium. Therefore, overall bone mass does not change. 16 , 24 , 25 , 26 In periodontitis, the impaired OB/OC balance occurs due to overt immune activity against the dysbiotic microbiome and shifts the equilibrium of bone metabolism toward pro‐resorption. 16 , 24 , 25 , 26 The prolonged exposure to virulence factors from oral biofilm components triggers an exuberant immune response which initiates molecular and cellular pathways activating alveolar bone loss. 27 , 28 , 29 , 30 , 31 , 32 Figure 2 shows the activation of uncontrolled innate immune responses that occur in periodontitis. Clinical manifestations of mucosal inflammation of gingival tissues are evident such as edema, redness, and bleeding due to the impaired intrinsic molecular mechanisms that control periodontal pathology. 33 , 34 , 35 Dysbiosis is not the only cause of periodontitis. Diverse non‐modifiable risk factors including age, gender, genetic/epigenetic networks (polymorphisms in immune pathway‐related genes, e.g., IL‐1A, IL‐1B, IL‐6, IL‐10, MMP‐3, MMP‐9) are significantly associated with the risk of developing periodontitis. Modifiable factors such as systemic inflammatory diseases (obesity, diabetes, and others), diet, environmental, and behavioral factors (e.g., smoking and stress) can further exacerbate the disease progression, severity, or the outcomes of periodontal treatments. 36 , 37
FIGURE 2.

Uncontrolled activation of innate immune response occurs in periodontitis. (1) Oral biofilm composed of periodontal pathogens induce oral dysbiosis. (2) Virulence factors released by dysbiotic bacteria such as, gingipain proteases (Porphyromonas gingivalis) and heat shock protein (Aggregatibacter actinomycetemcomitans) (3) recruit neutrophils to the inflamed gingiva resulting in uncontrolled pro‐inflammatory cytokine released by keratinocytes, dendritic cells, and fibroblasts, (4) inducing an overreactive innate immune response due to (5) the increased release of pro‐inflammatory cytokines and metalloproteinases (MMPs) by macrophages and (6) overactivation of CD4+T cells, involved in the polarization of T‐helper subtypes and B cells. (7) The uncontrolled increase in levels of pro‐inflammatory cytokines, MMPs and upregulation of TH1 and TH17 promote bone resorptive cells and induction of OC.
A well‐functioning periodontal homeostatic mechanism requires an efficient system of bone homeostasis supported by periodontal ligament cells, gingival fibroblasts, junctional epithelial cells, keratinocytes, neutrophils, macrophages (Mφ), dendritic cells (DC), and lymphocytes for the maintenance of a healthy periodontium 32 , 33 , 38 , 39 , 40 , 41 , 42 (Figure 2). However, in inflamed gingiva, dysbiosis‐induced metalloproteinase (MMP) activity accelerates collagen degradation in gingival connective tissue, junctional epithelium, and periodontal ligament leading to periodontal pocket formation accompanied by a significant increase in inflammatory cell infiltration. 34 , 35 , 43 , 44 Neutrophils located in the gingival sulcus are the first line of defense secreting α‐defensins to clear the colonizing periodontopathogenic bacteria. 45 , 46 , 47 , 48 Neutrophils phagocytose bacteria and contain copious amounts of reactive oxygen species (ROS) and antimicrobial agents stored in the granules driving pathogen clearance by intracellular killing. 49 , 50 In addition to these killing mechanisms, neutrophils deploy neutrophil extracellular traps (NETs), a network of extracellular fibers comprised of granule proteins and chromatin, aimed at preventing bacterial adhesion and invasion of host tissues. 51 , 52 NETs can be found within the purulent gingival crevicular exudate and play a vital role in the clearance of periodontal pathogens by trapping dispersed subgingival plaque. 53 In response to dysbiosis, neutrophils show delayed apoptotic activity with impaired antibacterial function and uncontrolled immune response. 45 , 46 , 47 In addition, keratinocytes release pro‐inflammatory mediators such as β‐defensins while fibroblasts, DC and keratinocytes secrete proinflammatory cytokines such as IL‐1β (Interleukin‐1 Beta), IL‐6 (Interleukin 6), IL‐23 (Interleukin 23), IL‐8 (Interleukin 8), and TNFα (Tumor Necrosis Factor Alpha) promoting recruitment of neutrophils, Mφ, and lymphocytes to the site of infection. This vicious cycle of autoinflammatory signal amplification, in turn, stimulates inflammatory Mφ to secrete copious amounts of pro‐inflammatory cytokines and MMPs that activate osteoclastogenesis and collagen degradation during disease progression. 54 , 55 , 56 , 57 , 58 DC perform a dual role as both phagocytes and antigen‐presenting cells (APCs). APCs activate CD4+ T cells (CD4 T lymphocytes or “helper T cells”) to various pathogenic effector phenotypes. 59 Cheng et al. 60 demonstrated that uncontrolled upregulation of Th1 (Type 1 T helper) and Th17 (Type 17 T helper) cell pathways also promote the induction of OC, giving rise to areas of future research. Further, monocytic myeloid‐derived suppressor cells (M‐MDSCs) are positively associated with T cell immunosuppressive and osteoclastogenic activity in the context of periodontal health and disease. 61 A high percentage of M‐MDSCs express macrophage colony‐stimulating factor (M‐CSF) receptor (c‐fms). In turn, this c‐fms+ subpopulation is considered an osteoclast precursor and in the presence of RANKL (Receptor activator of Nuclear Factor Kappa Ligand) and MCSF, this subpopulation can differentiate into osteoclasts and promote bone loss in periodontitis. 62
In summary, overt immune responses due to the upregulated pro‐inflammatory status drive impaired OB/OC crosstalk exacerbating periodontitis.
3. PERTURBATION OF OSTEOBLAST–OSTEOCLAST CROSSTALK OCCURS IN PERIODONTITIS
OB are of mesenchymal origin and their activation requires a sequential activity of transcription factors 17 , 63 , 64 , 65 (Figure 3; Box 1‐dark yellow color). For instance, Runx2 (osteoblast‐specific factor/core binding factor) gene activates the expression of osteocalcin (OCN), bone sialoprotein (BSP), osteopontin (OPN), and collagen synthesis to ultimately activate the expression of bone‐related proteins such as bone morphogenetic protein (BMP‐2) and transforming growth factor beta (TGF‐β). 17 , 63 In conjunction with Runx2, osterix (OSX) plays a key role in periodontal tissue remodeling, including differentiation and formation of OB and cementoblasts. OSX also regulates the expression of OCN, osteonectin (ON) and BSP, genes that regulate OB activity, collagen binding, and ECM calcification. 66 , 67 Transcription factor Krüppel‐Like Factor 4 (KLF4) promotes dentinogenesis and odontoblastic differentiation by modulating the TGF‐β signaling pathway, while Forkhead box protein O1 (FoxO1) has been reported as a crucial mediator in bone homeostasis by providing a favorable environment for OB proliferation and differentiation 68 (Figure 3; Box 2‐Dark blue color). Conversely, Wnt acts on OB precursor cells to promote their differentiation into mature OB through the β‐Catenin‐dependent canonical pathway. However, the observed dysregulation of the Wnt/β‐Catenin pathway is a critical aspect during the progression of periodontitis 69 , 70 , 71 (Figure 3; Box 3‐brown color). In addition to TFs, non‐protein signaling molecules like nitric oxide (NO; a free radical mediator) and prostaglandin E2 (PGE2; a lipid mediator) also contribute to bone metabolism by regulating RANKL pathway 72 (Figure 3; Box 4‐red color). The biological effects of NO on bone metabolism vary based on concentration. For instance, low NO may support osteoblast bone formation and OC‐mediated bone remodeling, while at higher concentrations, NO inhibits OC formation and bone resorption. 73 RANKL activates inducible nitric oxide synthase (iNOS) to enhance NO levels in OC thereby inhibiting osteoclastogenesis. 74 PGE2 induces RANKL expression in OB in an autocrine and paracrine manner via activation of the prostaglandin E2 receptor 4 (EP4), exerting regulatory action on angiogenesis and vascular permeability thus modulating bone metabolism. 75
FIGURE 3.

Uncoupling mechanism between osteoclasts and osteoblasts in periodontitis. The downregulation of OPG promotes an enhanced interaction of RANK and RANKL resulting in OC differentiation and activity. Pro‐inflammatory cytokines (IL‐1, IL‐11), MMPs, hormones (PTH), vitamin D3, inflammatory lipids (PGE2) can also promote OC differentiation and bone resorption. In addition, OB/OC communication by EFNB2‐EFNB4, FAS–FASL, and NRP1‐SEMA3A are disrupted, promoting altered alveolar bone homeostasis in periodontitis.
OB exhibit autocrine and paracrine activity that regulates activation of osteogenic as well as osteoclastic factors. Indeed, BMPs and TGF‐β control autocrine activity by promoting differentiation and activation of OB (Figure 3; Box 5‐green color), while colony‐stimulating factor‐1 (CSF‐1), granulocyte colony‐stimulating factor (G‐CSF), basic fibroblast growth factor (basic FGF), insulin‐like growth factor (IGF), and RANKL control paracrine activity involved in OC differentiation and activation 17 , 76 , 77 (Figure 3; Box 6‐dark pink color). In periodontitis, the autocrine function of OB is inhibited favoring differentiation and activation of OC due to OB‐associated paracrine function. 78 , 79
OC are multi‐nucleated, bone‐resorbing cells of myeloid origin. Activation of OC is regulated by the expression of IL‐1β, IL‐6, IL‐23, TNFα, and specific MMPs secreted by Mφ (Figure 3; Box 7‐ gray color). This facilitates the interaction of RANKL (expressed on OB) with its receptor RANK (Receptor of Nuclear Factor‐kappa) (expressed on OC precursors), promoting the differentiation and activation of mature OC 80 , 81 , 82 , 83 (Figure 3; Box 8‐gold color). In brief, the binding of M‐CSF to its receptor, c‐Fms, induces the transcription factor c‐Fos, whereas the binding of RANKL to its receptor, RANK, leads to the recruitment of TNF‐receptor‐associated factor 6 (TRAF6), the main adapter molecule of RANK. TRAF6 activates nuclear factor κB (NF‐κB) and mitogen‐activated kinases (MAPKs) including c‐Jun N‐terminal kinase (JNK). In turn, JNK activates the transcription factor c‐Jun. RANKL/RANK also induces c‐Fos to form activator protein 1 (AP‐1), a heterodimeric transcription factor, which along with c‐Jun. AP‐1 and NF‐κB then induce the nuclear factor of activated T cells 1 (NFATc1), a master transcription factor that regulates OC differentiation. NFATc1 works with other transcription factors, such as AP‐1, PU.1, and microphthalmia‐associated transcription factor (MITF) to induce various osteoclast‐specific genes. 84 Conversely, expression of osteoprogeterin (OPG), a RANKL decoy that prevents differentiation and activation of OC, is downregulated in periodontitis. This favors interaction between RANKL and RANK receptor to promote OC differentiation and maturation. Similarly, OPN and ON expression are downregulated in periodontitis suggesting reduced OB cell activity. 84 , 85 , 86 , 87 Furthermore, parathyroid hormone (PTH) is secreted by the parathyroid glands and contributes to the maintenance of calcium levels in the blood (Figure 3; Box 9‐ Light yellow color). The upregulation of PTH or continuous treatment with PTH has been associated with an increase in OC number and activity due to an altered RANKL/OPG ratio. 88 Vitamin D3 possesses anti‐inflammatory properties and plays an essential role in calcium regulation and bone metabolism. In periodontitis, downregulation of vitamin D3 is associated with an increase in OC‐associated bone resorption. 89 Prostaglandin (PG) E2 (PGE‐2) and IL‐1β are important bone remodeling mediators. However, their upregulation stimulates the production of MMPs in myeloid cells and oral epithelial cells which in turn, induces RANKL expression in OB, promoting the differentiation and activation of OC via RANKL/RANK interaction. 90 , 91 Paradoxically, IL‐11 exerts pro‐inflammatory effects by triggering periodontitis in the earlier stages of the disease but in the advanced stages of disease, an anti‐inflammatory role for this molecule has been observed. Indeed, in a dog model of periodontitis, treatment with IL‐11 resulted in the resolution of periodontal inflammation by downregulating the expression of pro‐inflammatory cytokines such as IL‐6, IL‐8, and TNFα. 92
Synchronized communication between OB/OC is essential for bone homeostasis 21 , 22 (Figure 3; Box 10‐Light blue color). Ephrin2‐Ephrin4 (EFNB2‐EPHB4), FAS–FASL, and NRP1‐SEMA3A (Neuropilin‐1‐Semaphorin 3A) are the key molecules that fine‐tune OB/OC crosstalk. 93 , 94 EFNB2 and EPHB4 are expressed on the cell surface of OB and OC, respectively, and are bidirectionally involved in bone remodeling. OB differentiation is promoted by EFNB2‐mediated activation of EPHB4. However, the reverse signaling EPHB4‐mediated activation of EFNB2 suppresses OC differentiation. 93 The efficient crosstalk between OC‐expressed FAS and OB‐expressed FASL plays an integral role in regulating apoptosis in FAS‐expressing cells, and their dysregulation is associated with tumorigenesis and auto‐immune diseases. Estrogens trigger the upregulation of FASL expression in OB resulting in apoptotic pre‐OC. However, in estrogen‐deficient mice, the downregulation of FASL expression resulted in reduced OC apoptosis associated with increased levels of bone resorption. 94 NRP‐1‐SEMA3A expressed in OC and OB, respectively, has a role in alveolar bone homeostasis. Indeed, their interaction inhibits RANKL‐induced OC differentiation and promotes OB differentiation through the Wnt/β‐catenin pathway. 95 Notch signaling pathways and their regulators (Notch1‐3) are key factors in periodontitis. 96 , 97 In healthy gingiva, Notch signaling controls OC differentiation and bone‐resorbing activity by targeting OC precursors and/or acting directly on OB by activating their differentiation. NOTCH1 inhibits OC through the RBPJk (recombination signal binding protein for immunoglobulin kappa J region) canonical pathway, and by enhancing OPG levels and Wnt signaling in OB and osteocytes (Figure 3; Box 11‐Light pink color). NOTCH2 induces OC through NF‐κB and HES1‐dependent (hairy and enhancer of split 1) mechanisms and by increasing RANKL in OB, while NOTCH3 promotes OC by enhancing RANKL levels in OB and osteocytes 98 (Figure 3; Box 12‐purple color). In periodontitis, a significant increase in NOTCH2 (exerting pro‐OC function), TNF‐α, IL‐17, and RANKL was observed, while Notch1 and Jagged1 levels were decreased compared to healthy controls. Higher levels of TNF‐α and RANKL contribute to increased alveolar bone resorption by increasing the expression of RANK in OC precursors and RANKL expression in OB. 96 , 97 In summary, impaired crosstalk between OB and OC impacts bone homeostasis due to dysfunctional OB proliferation, differentiation, and survival. 21 , 22 , 25
Uncontrolled bone resorption by OC occurs in periodontitis (Figure 3; Box 13‐orange color). In brief, the first step of bone resorption is the acidification of bone matrix composed of more than 90% type I collagen (osteoid) and hydroxyapatite (bone mineral composed of calcium and phosphate crystals). 96 , 97 , 98 During the bone resorption process, OC forms unique cytoskeletal structures termed the sealing zone (SZ) and ruffled border (RB) that are essential to the bone resorption process. 96 Once the SZ is attached to bone surface, vacuolar H+‐adenosine triphosphatase (H + ‐ATPase) located on the RB membrane secrete H+ to acidify the resorption lacuna formed underneath the RB. 74 , 96 , 98 To counter the acidic pH driven by H+‐ATPase, the chloride voltage‐gated channel 7 (CLCN7), also located in the RB, secretes Cl− to maintain electroneutrality. 96 , 97 , 98 Thereafter, cathepsin k (CTSK), a member of the papain superfamily of cysteine protease, degrades the organic component of bone. 96 , 97 , 98 Along with CTSK, MMPs, DC‐specific transmembrane protein (DC‐STAMP), tartrate‐resistant acid phosphatase (TRAP), and OC‐associated receptor (OSCAR) regulate the final steps of bone degradation. 96 , 97 , 98 , 99 , 100 Degraded products generated during this process are endocytosed from resorption lacuna and released through the apical membrane of the OC. 96 , 97 , 98 , 99 , 100 Once the bone resorption process is completed, apoptotic signals are activated in OC or a new bone resorption cycle ensues. 96 , 97 , 98 , 99 , 100 In periodontitis, impaired OC activation triggers additional and irreversible alveolar bone resorption cycles due to the overt dysbiosis‐driven immune response. 13 , 14 , 15 , 43 In summary, the understanding of immunopathology and osteobiology of periodontitis is essential for developing novel treatment protocols.
4. THE EVOLUTION OF PERIODONTITIS TREATMENT FROM CONVENTIONAL TREATMENTS TO CURRENT NON‐SURGICAL PERIODONTAL THERAPY (NSPT)
Alveolar bone loss is a hallmark of periodontitis, and its prevention is a key clinical challenge in successful management of the disease. 1 , 2 , 3 , 7 , 8 , 9 , 24 , 25 Current treatment strategies focus on controlling adverse systemic health factors (ex. poor glycemic control for diabetics), behavior modification (smoking cessation, establishing a good oral hygiene habit, etc.), and mechanical plaque biofilm and calculus debridement. In advanced stages of disease, periodontal surgery is employed; however, the discussion of which is beyond the scope of this article. Excellent reviews have been published on the subject. 101 Unfortunately, these approaches do not always restore bone homeostasis and often result in limited success. 102 Recurrence of the disease is also common. Therefore, the development of novel therapeutic approaches is imperative to prevent overt periodontal inflammation and the cascade of events leading to periodontitis.
4.1. Treatment protocols to treat periodontitis
Importantly, mechanical debridement (scaling and root planing (SRP), “deep cleaning”) with or without the use of adjunctive antimicrobials such as systemic antibiotics (amoxicillin, metronidazole, azithromycin, and doxycycline) or topical (sulcular) application of antimicrobial agents (minocycline microspheres and chlorhexidine chip) is a viable therapeutic approach aimed at eliminating etiological factors of disease and initiating resolution of periodontal inflammation. 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 Randomized clinical trials have indicated that treatment with amoxicillin and metronidazole adjuncts to SRP demonstrates a significant improvement in clinical outcomes. 111 , 112 , 113 Unfortunately, non‐surgical therapeutic approaches have not changed significantly over the last 2–3 decades and depend on the removal of etiological factors in a closed environment relying on the tactical feel of calculus, rather than visualization, which can be challenging. In advanced cases, periodontal surgery using resective or reconstructive approaches is indicated. 100 , 101 In brief, resective periodontal surgery is based on the principles of surgical exposure, removal of etiological agents, recontouring of affected alveolar bone to create physiologic bony contours, and removal of excessive soft periodontal tissue from periodontal pockets. 114 Although this approach recreates a more favorable periodontal environment, it does not result in a gain of clinical attachment level (CAL), so a gingival recession is common. Alternatively, reconstructive (regenerative) periodontal surgery utilizes bone graft materials (autogenous, allograft, xenograft, alloplastic), membranes (resorbable, non‐resorbable), and/or biomimetics (PDGF‐BB, amelogenins, etc.) to regenerate damaged periodontal tissues and CAL. 115 Unfortunately, regenerative periodontal therapies are not always successful for advanced cases of disease, and specific periodontal treatment planning is required for different individuals. Therefore, new therapeutic strategies are needed to prevent or restore bone alveolar homeostasis at the earlier stages of the disease without the need for periodontal surgery. This shift toward prevention or restoration of alveolar bone homeostasis in periodontitis will unravel new research avenues in the field.
4.2. Overview of alternative non‐surgical therapeutic strategies for periodontitis
Concerns regarding antibiotic resistance have promoted new research and clinical investigation of alternative periodontal treatment strategies. Indeed, novel therapeutic approaches are oriented toward the identification of key molecular targets whose inhibition or activation results in reduced periodontal inflammation and return to physiologic alveolar bone homeostasis. New therapeutic adjuncts to SRP have been proposed as alternate treatment strategies to target inflammatory mediators and bone‐resorbing osteoclasts and are discussed below.
4.2.1. Bisphosphonates
Bisphosphonates are chemically stable derivatives of inorganic pyrophosphate (PPi) with anti‐resorptive properties that impair OC differentiation and activity thus inhibiting bone resorption 116 , 117 , 118 (Figure 4A). Bisphosphonates target mature OC by inhibiting farnesyl diphosphate synthase (FPP) impairing OC function and driving apoptosis. 116 Commonly used bisphosphonates to treat osteolytic diseases such as osteoporosis include zoledronate and alendronate. 116 , 117 , 118 Although oral and topical treatment with bisphosphonates has achieved successful outcomes in periodontitis patients, adverse clinical events have been noted. Osteonecrosis of the jaw (ONJ) is a result of prolonged use of bisphosphonates promoting an area of exposed alveolar bone, delayed wound healing, and necrosis of affected bone. 119 Thus, the risks and benefits of periodontal surgery should be carefully considered in these patients. In a rat model of ligature‐induced periodontitis (LIP), treatment with alendronate (2.5 mg/kg) resulted in reduced OC activity along with reduced alveolar bone loss but after 21 days of treatment, ONJ manifestations in the maxilla region were indicative of adverse biological effects of prolonged drug treatment. 120 Conversely, another LIP study in rats treated with alendronate resulted in beneficial clinical outcomes exhibiting reduced inflammation and robust tissue repair. 121 To support preclinical observation, numerous clinical trials have been published over the last decade. Bisphosphonates as adjuncts to SRP have demonstrated expected clinical outcomes for short‐term periodontitis therapy, but concerns regarding ONJ limit their long‐term clinical use. 120 Rocha et al. (2001), evaluated the effect of alendronate (10 mg/daily) for 6 months in a short‐term randomized controlled pilot trial with patients diagnosed for diabetes and established periodontitis. This RCT showed an improvement in the alveolar bone loss by decreasing the cementoenamel junction (CEJ) to alveolar bone crest distance. No metabolic parameter changes were noted at any time during the duration of the RCT. 122 In line with this, an RCT in postmenopausal women (without hormone replacement therapy) tested the efficacy of alendronate (10 mg/day) on periodontal disease. After 6 months of treatment with alendronate, the bone turnover ratio was improved in postmenopausal women with established periodontitis by reducing the BOP and gingival bleeding without any observed changes in the hormone levels. 123 Lane et al. (2005) evaluated the efficacy of bisphosphonate therapy as an adjunct to SRP and 3‐month periodontal maintenance therapy in patients with moderate‐to‐severe chronic periodontitis. After 1 year, the SRP + bisphosphonate group demonstrated greater clinical improvements in BOP, CAL, and probing depth (PD) compared with the SRP alone. 124 Graziani et al. (2009) observed similar clinical outcomes (BOP, CAL, and PD) in an RTC testing the efficacy of the bisphosphonates and SRP in patients with moderate‐to‐severe chronic periodontitis. This RCT indicated that bisphosphonates may be an appropriate adjunctive treatment by preserving the periodontal bone mass. 125 Sharma et al. (2012) tested the efficacy of local delivery of 1% alendronate gel as an adjunct to SRP in chronic periodontitis patients for 6 months. The RCT results showed that local delivery of 1% alendronate into the gingival sulcus of diseased sites led to a significant decrease in PD and BOP and a gain in CAL compared to the placebo group. 126 Similarly, patients with chronic periodontitis and type 2 diabetes (T2D) treated with local delivery of a 1% alendronate gel as an adjunct to SRP showed improvements in the bone mass‐related parameters (decreased PD, BOP, and gain in CAL). 127 While the results presented above seem promising, a systematic review by Alwithanani 128 compiled the RCTs mentioned above and concluded that although bisphosphonate therapy is a useful adjunct to periodontal treatment, the practical application of these agents in clinical practice is tempered by the possibility of developing ONJ. Furthermore, these drugs are not metabolized but are either excreted renally or deposited within bone and remain in the body for decades. The longest duration of placebo‐controlled clinical trials is 6 years and subjects in these observational studies have used the newer amino‐bisphosphonates for 10 year. Of note, there is no known method of removing these agents from bone tissues. As such, these medications exhibit profound effects on bone physiology that are not well recognized, along with uncertain long‐term consequences. 129 Further studies detailing the safety and efficacy of bisphosphonate therapy are required before clinical recommendations can be made.
FIGURE 4.

Anti‐resorptive non‐surgical therapy adjuncts to scaling root and planing. The anti‐resorptive drugs: (A) Bisphosphonates, (B) OPG‐Fc and RANKL inhibitors, and (C) Strontium Ranelate, adjuncts to SRP inhibit OC differentiation and activity, driving a reduced bone resorption. However, some adverse clinical events have been observed due to long‐term administration of these drugs. ONJ is observed in long‐term treatment with bisphosphonates, OPG‐Fc, and RANKL inhibitors. To date, no ONJ cases have been reported during SR treatment.
4.2.2. OPG‐Fc and RANKL Inhibitors
OPG and RANKL are key molecules regulating the differentiation and activation of mature OC. 81 , 82 , 83 , 84 , 85 , 86 As mentioned above, the OPG/RANK/RANKL axis is impaired in periodontitis, driving a shift from OPG/RANKL to RANK/RANKL interaction and promotion of OC differentiation and maturation. 81 , 82 , 83 , 84 , 85 , 86 The discovery of these molecules and their crosstalk have spurred the development of new therapeutic strategies targeting periodontitis in both animal models and human clinical trials. 130 , 131 , 132 , 133 , 134 , 135 Recent in vivo studies have revealed the therapeutic potential of OPG. Teng et al. 130 observed significant inhibition of alveolar bone resorption by targeting OPG. Indeed, mice inoculated with the periodontal pathogen A. actinomycetemcomitans and treated with OPG showed reduced alveolar bone loss associated with a decreased number and activity of mature OC 130 (Figure 4B; upper panel). Denosumab, an anti‐RANKL monoclonal antibody, has been tested to treat osteoporosis and metastatic bone cancer 131 (Figure 4B; lower panel). It inhibits RANKL by mimicking OPG expressed in OB and regulates differentiation and activation of OC. 12 , 133 In periodontitis, bone turnover is disrupted due to overt bone resorption by OC. 16 , 24 , 25 , 26 Denosumab treatment restores bone turnover similar to that observed with bisphosphonate therapy; however, shorter half‐life of Denosumab (generally 6 months or less) provides a pharmacological window that allows for safer surgical procedures, such as dental extractions or flap surgeries, compared to the long‐lasting effects of bisphosphonates, which can complicate such treatments. 132 However, similar to bisphosphonate therapy, adverse events such as denosumab‐related ONJ (DRONJ) have been reported. 133 , 134 A retrospective study showed that DRONJ is related to many risk factors such as invasive dental procedures (tooth extraction or dental implant surgery), poor oral hygiene, corticosteroids, smoking, anemia, diabetes, or renal failure. Identification of these risk factors in periodontitis patients may be a diagnostic marker for DRONJ. 135 Perhaps, such drugs can be used as personalized dental treatments after further study.
4.2.3. Strontium ranelate
Another recent antiresorptive drug used in osteoporotic menopausal women is strontium ranelate (SR), composed of strontium salt of ranelic acid. SR is a dual‐action bone agent with concomitant antiresorptive and osteoanabolic properties which represents an advantage over bisphosphonates 136 , 137 , 138 , 139 , 140 , 141 , 142 (Figure 4C). In brief, one of the mechanisms of action results from the interaction of strontium cations (Sr2+) with extracellular calcium‐sensing receptors (CaSRs) on mature OC‐inducing apoptosis. Concurrently, SR impacts CaSRs expression on OB leading to the activation of bone‐forming signaling pathways, such as TGFβ, BMPs, etc., driving the proliferation of new OB. 139 Additionally, SR increases the expression of OPG and concomitantly reduces RANKL expression in OB. 137 Karakan et al. (2017) tested three different doses of SR (300, 625, and 900 mg/kg) administered daily by oral gavage in a rat model of periodontitis for 11 days after ligature placement. Interestingly, SR treatment decreased bone loss, which was attributed to reduced numbers of OC and a concomitant increase in OB. 140 Despite the contrasting effects, SR is a potent inhibitor of OC as observed by reduced alveolar bone loss in experimental animal models of periodontitis. 140 , 141 , 142 Controlled clinical studies in humans is required before SR can be used as a therapeutic adjunct.
4.2.4. CTSK inhibitors
Oral antiresorptive therapies have yielded new approaches targeting impaired host immunity in periodontitis. 143 CTSK is a member of the papain‐like cysteine protease family playing a role in the innate immune response and regulating OC‐mediated bone resorption. 144 After OC activation, CTSK is released into the resorption lacuna leading to bone resorption. 96 , 97 , 98 CTSK degrades type I collagen fibers (90% of the bone organic matrix) and cleaves and activates MMP9 leading to type II collagen degradation. 96 , 97 , 98 CTSK is recognized as an important bone resorption marker and potential therapeutic target. 144 CSTK inhibitors are among the most promising immunomodulatory drugs with the potential to restore alveolar bone homeostasis in periodontitis. 144 , 145 Inhibition of CTSK regulates bone resorption by inhibiting OC activity rather than reducing OC viability and without affecting OB activity, thereby restoring alveolar bone turnover. 146 Numerous CTSK inhibitors are currently undergoing human clinical trials for the treatment of multiple diseases including osteoporosis, autoimmune disorders, metabolic diseases, and others. However, adverse events such as cardiovascular and cerebrovascular accidents have been reported 146 , 147 (Figure 5). In experimental periodontitis, the use of CTSK inhibitors has shown beneficial outcomes. 148 , 149 , 150 , 151
FIGURE 5.

Use of Cathepsin K (CTSK) inhibitors to prevent or treat periodontitis. Balicatib, Odanacatib, adeno‐associated virus‐sh‐CTSK (AAV‐sh‐CTSK), BML‐224 and CsinCPl‐2 are therapeutic strategies targeting CTSK to treat periodontitis. Balicatib and Odanacatib have been tested to treat osteoporosis with insightful clinical outcomes reducing bone resorption. However, osteonecrosis on the jaw (ONJ) cases has been reported in Balicatib treatment and cardiovascular and cerebrovascular events have been observed in Odanacatib treatment. Odanacatib treatment in a mice model of periodontitis has resulted in positive outcomes by targeting innate immune signaling pathways such as TLR4, 5, and 9 pathways. Other experimental drugs targeting CTSK, such as AVV‐sh‐CTSK, BML‐224, and CsinCPl‐2, have reported therapeutic value to treat periodontitis by reducing inflammation and bone‐resorptive cell activity.
Balicatib (formerly AAE581, Novartis) is a CTSK inhibitor with high selectivity properties targeting cathepsin B, L, and S. Balicatib was tested in ovariectomized monkeys over 1 year of treatment. 152 The anti‐osteoclastic activity was reflected by an increase in the expression of bone markers and bone mineral density in the spine, femur, and hips. In a phase I clinical trial, a 25 mg dose was well tolerated in ovariectomized monkeys inducing significant CTSK repression and new bone formation. 153 In postmenopausal women with osteopenia/osteoporosis, a clinical trial was discontinued at phase II due to the cutaneous lesions, such as morphea‐like skin or skin rashes and adverse pulmonary events. 154 To date, Balicatib treatment has not been reported in periodontitis models.
Odanacatib (formerly MK‐0822, Merck & Co.) is a CTSK inhibitor with high selectivity for cathepsin K. 155 Odanacatib was tested in ovariectomized monkeys with successful outcomes as observed by significant bone formation without any negative impact on bone turnover. 156 Odanacatib reached phase III clinical trials in a randomized, double‐blind study with 16 713 osteoporotic postmenopausal women using a 50 mg dose once weekly for 3 years. 156 Although long‐term treatment with Odanacatib during phase III of the clinical trial increased bone mineral density, trabecular volumetric, and strength of spine and hip compared with placebo, it was discontinued due to an increase in cerebrovascular accidents and adverse cardiovascular events. ONJ, adverse respiratory infections, or systemic sclerosis were not noted. 157 , 158 , 159 Wild‐type female BALB/cj mice challenged with P. gingivalis to induce periodontitis for 56 days and treated with Odanacatib showed a decrease in the numbers of OC, Mφ, and T cells. 151 , 152 , 160 Thus, Odanacatib exhibits immunomodulatory properties in conjunction with its anti‐resorptive activity. Indeed, a significant inhibition of downstream Toll‐like receptor (TLR) signaling pathways was observed in gingival epithelial cells indicating that Odanacatib regulates the innate immune response in periodontitis. 160
Other recent experimental treatments inhibiting CSTK expression include injections of small interfering RNA (siRNA), adeno‐associated virus (AAV), CTSK‐specific inhibitors, BML‐244, or CsinCPI‐2 highlight the therapeutic potential of CTSK inhibitors to treat periodontitis. 161 , 162 , 163 Chen et al., 164 showed that AAV expressing small interfering RNA (siRNA) (AVV‐sh‐CTSK) silenced CTSK expression in a mice model of periodontitis induced by oral gavage with Pg. Local Injections of AVV‐sh‐CTSK into the palatal gingiva for 7 days showed a significant reduction in gingival inflammation and less alveolar bone resorption. In a double mice model of rheumatoid arthritis (RA) induced by collagen and Pg infection‐induced periodontitis, respectively, injections of AAV containing siRNA‐CTSK in the knee joint and inflamed gingiva resulted in a significant reduction of inflammation in the synovium and alveolar bone loss, respectively. Another study conducted by Pan et al. 160 using a combinatorial periodontitis and RA model treated with the CTSK inhibitor, BML‐224 showed similar outcomes including anti‐inflammatory responses and reduced alveolar bone loss. Da Ponte Leguizamón et al. 161 demonstrated in vivo therapeutic effects of the phytocystatin CsinCPI‐2, derived from Citrus sinensis. In a murine LIP model, systemic treatment of CsinCPI‐2 resulted in a reduced inflammatory response and less bone resorption due to inhibition of OC differentiation and activation as reflected by low RANKL‐induced TRAP+ cells. 161
A key limitation of CTSK inhibitors is that expression of CTSK is not restricted to bone‐forming cells. Thus, the use of these CTSK inhibitors has been associated with a poor diagnosis of metabolic and immunoregulatory processes. 150 , 151 , 152 Importantly, the dual function of CTSK inhibition, anti‐inflammatory and anti‐resorptive, suggests a promising therapeutic strategy. While CTSK inhibitors show promise in treating osteoporosis and other bone diseases by inhibiting bone resorption, their development has been challenged by safety concerns. Further research is needed to overcome these challenges and to develop safe and effective CTSK inhibitors. 162
4.2.5. Specialized pro‐resolving mediators
Specialized pro‐resolving mediators (SPM) are endogenous immunoregulatory lipid molecules that play crucial roles in the resolution of periodontal inflammation. 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 SPM are oxylipids mediators, including resolvins, maresins, lipoxins, and protectins, derived from omega‐3 polyunsaturated fatty acid. 163 , 164 , 165 Experimental studies with SPM have demonstrated enhanced gingival tissue repair and clearance of oral pathogens (Figure 6). This included robust innate and adaptive immune responses by promoting monocyte infiltration, activation of Mφ, inhibition of neutrophil infiltration, and cytokine production driving the resolution of inflammation. 163 , 164 , 165 , 166 Indeed, E‐series resolvins (RvE1) interact with BLT1 expressed on neutrophils and chemR23 expressed on Mφ, monocytes, and DC inhibiting their infiltration and cytokine production. 166 , 167 The main function of SPM is to restore tissue homeostasis and recent animal studies have shown its potential therapeutic value in periodontitis treatment. For instance, LIP transgenic mice overexpressing human chemR23 (Gao et al., 2013), LIP male Wistar rats (Lee al., 2016), and studies with LIP male rabbits followed by Pg infection (Hasturk et al., 2006; Hasturk et al., 2007) treated with local RvE1 resulted in reduced inflammatory bone resorption associated with a reduced OC activity. 168 , 169 , 170 , 171 Hasturk et al. (2021) tested the efficacy of Lipoxin A4 (LXA4), an SPM that acts via GPC‐receptor, and observed a significant reduction in inflammation in patients with gingival inflammation by modulating inflammatory immune pathways. The phase 1 RCT indicated that after 28 days of treatment with an oral rinse containing a LXA4 mimetic, methyl ester‐benzo‐lipoxin A4 (BLXA4), the level of gingival inflammation was substantially reduced along with a reduction of periodontal pocket thereby suggesting LXA4 as a potential novel therapeutic treatment for periodontitis. 172 Mizraji et al. 173 tested the efficacy of RvD2 in BALB/c mice in a Pg‐induced experimental periodontitis model for 8 weeks and observed significantly reduced inflammation and alveolar bone loss in mice with experimental periodontitis. Qualitative assessment of the above‐mentioned animal studies 168 , 169 , 170 , 171 , 172 , 173 was performed by a systematic review using the ARRIVE guidelines (arriveguidlines.org) 174 and noted that Resolvins demonstrate efficacy in the treatment of periodontitis by inhibiting the destructive inflammatory process and loss of alveolar bone in laboratory‐induced periodontitis under controlled experimental conditions. 174
FIGURE 6.
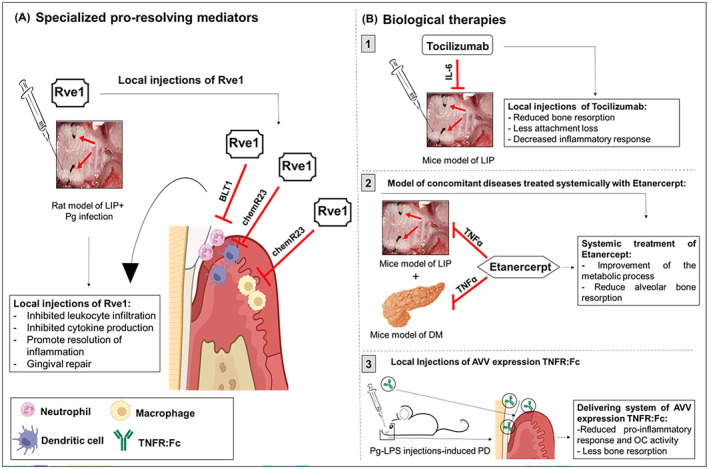
Immunomodulatory therapy adjuncts to scaling root and planing. Non‐surgical immunomodulatory therapies: (A) Specialized pro‐resolving mediators (SPMs) and (B) Biological therapies targeting IL‐6, TNFα improves alveolar bone homeostasis by decreasing inflammation, regulating immune responses, and reducing OC activity.
4.2.6. Biological therapies
Inhibitors of pro‐inflammatory mediators TNFα, IL‐6, IL‐1β regulating inflammatory responses have been tested in experimental models of periodontitis 175 , 176 , 177 , 178 , 179 (Figure 6). A recent study analyzed the anti‐inflammatory effects of tocilizumab, a monoclonal antibody targeting IL‐6, in a mice model of LIP. Results demonstrated a significant reduction in bone resorption, gain of tooth attachment, and reduced inflammatory responses. 176 Multiple studies have demonstrated that TNFα, a key signaling modulator of OC activation in periodontal pathology, is an excellent therapeutic candidate. 177 , 178 , 179 Indeed, two studies by the same group evaluated the effects of blocking TNFα activation by systemic administration of Etanercept in mice with concomitant diabetes mellitus (DM) and periodontitis. 177 , 178 After 5 weeks of treatment, results indicated that anti‐TNFα treatment improved the metabolic process in mice with DM and reduced alveolar bone resorption. Another feasibility study in a rat model of Pg LPS‐induced periodontitis, treated with AAV expressing TNF receptor immunoglobulin Fc (TNFR:Fc), reported reduced pro‐inflammatory responses and OC activity. 178 Similar results were observed in another study using pentoxifylline as a TNFα blocker in a mice model with concomitant arthritis‐associated periodontitis. 179 Treatment with this drug demonstrated reduced joint inflammation, reduced pro‐inflammatory responses, decreased infiltration of neutrophils, and diminished OC activity. Since periodontitis is a complex chronic inflammatory disease often associated with other chronic comorbidities, a combinatorial use of inhibitors targeting different inflammatory cytokines may yield better clinical outcomes, specifically in the later (moderate–severe) stages of the disease. Finally, it is important to evaluate systemic side effects or significant adverse events associated with inhibitors of pro‐inflammatory mediators in mice models of periodontitis. However, new approaches based on site‐restricted biologics, which do not affect immune activity in other non‐target tissues/organs, are highly desirable.
4.2.7. Non‐coding RNAs
Dysregulation of non‐coding RNAs targeting key osteolytic genes may contribute to the impairment of alveolar bone homeostasis and the progression of periodontitis. Multiple studies have shown the potential therapeutic benefits of targeting microRNAs (miRNA) in in vitro and in vivo models of periodontitis. 180 , 181 , 182 Epigenetic regulation by miRNA can modulate target gene expression through post‐transcriptional mechanisms. 181 , 182 , 183 , 184 Several miRNAs have been reported to exhibit immunomodulatory functions in the pathobiology of periodontitis and its progression, along with a promising value as diagnostic markers 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 ; Figure 7. Multiple reports have demonstrated a pathophysiologic role of miR‐146 in periodontitis, where levels are significantly increased compared to healthy gingiva. 184 , 185 , 186 , 187 Mechanistically, overexpression of miR‐146 is correlated with a significant decrease of pro‐inflammatory cytokines, IL‐1β, IL‐6, IL‐8, and TNFα by targeting NF‐κB. 184 , 185 , 186 , 187 Reduction in the levels of pro‐inflammatory cytokines attenuates the differentiation of bone‐resorbing cells. 186 Thus, miR‐146 acts as a negative feedback regulator of periodontal inflammation and may be of significant therapeutic value to be further tested in future clinical trials. 187 Similarly, miR‐21 and miR‐200c have been shown to exert anti‐inflammatory and anti‐osteoclastic functions by suppressing NF‐κB signaling and RANKL/OPG. 188 , 189 , 190 Conversely, miR‐223, a hematopoietic cell‐specific miRNA, has been reported to play a role in alveolar bone loss in periodontitis. 191 High levels of miR‐223 promoted differentiation and recruitment of neutrophils and osteoclastogenesis while inhibiting OB differentiation by targeting nuclear factor 1‐A (NF1‐A). 191
FIGURE 7.

Therapeutic targeting of non‐coding RNAs in periodontitis. miR‐146 and miR‐21 have an anti‐inflammatory role in mice models of periodontitis. Both target NFκB, reducing pro‐inflammatory cytokine expression and preventing OC differentiation and activation. miR‐200c exerts an anti‐inflammatory role in a rat model of periodontitis by targeting IL‐6, IL‐8, and Interferon‐1 related development regulator (IFRD1) and by reducing the RANKL/OPG ratio. miR‐223 has an inflammatory role in periodontitis. High levels of pro‐inflammatory miR‐223 impair bone turnover by inhibiting bone‐forming cells and promoting OC differentiation and activity. miR‐26a‐5p has an anti‐inflammatory role in human‐inflamed gingiva. High levels of miR‐26a‐5p are associated with resolution of inflammation by targeting PLCβ1 which is associated with the production of pro‐inflammatory cytokines. Mice model of LIP induces the overexpression of Malat‐1 and downregulation of miR‐30 expression by Malat‐1 sequestration. MALAT‐1 is upregulated while miR‐30b, an anti‐inflammatory miRNA, is downregulated causing induced expression of pro‐inflammatory macrophages, impaired coordinated innate immune responses, and unsuccessful resolution of inflammation.
Expression of miR‐142‐3p is downregulated in inflamed gingiva. 192 , 193 , 194 , 195 Valverde et al., 194 demonstrated that miR‐142‐3p targets multiple genes (including Vinculin, Dab2, and Skap2) that regulate cytoskeletal rearrangement and cell movement. In vivo, delivery of miR‐142‐3p in a murine LIP model showed reduced pro‐inflammatory cytokine (IL‐6 and TNFα) levels, neutrophil infiltration, and Th17 polarization [Valverde et al., Unpublished results]. In subjects with periodontitis, miR‐26a‐5p is downregulated in inflamed gingiva, while its cognate mRNA target PLCβ1 expression was upregulated compared to healthy control subjects. After non‐surgical therapy (SRP), expression of miR‐26a‐5p and PLCβ1 returned to levels observed in healthy subjects and correlated with improved clinical parameters suggesting their diagnostic and prognostic value. miR‐26a‐5p targets pathways related to host defense and wound healing, which are critical in periodontal pathogenesis and its resolution. 195
Besides miRNAs, long noncoding RNAs (lncRNAs), which are defined as regulatory non‐protein coding RNAs longer than 200 nucleotides, have recently gained attention in periodontal pathobiology. 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 LncRNAs regulate transcriptional, post‐transcriptional, translational, and epigenetic changes by physically interacting with DNA, RNA, miRNA, or proteins. 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 Several studies have indicated a pathological role of lncRNA in chronic inflammatory diseases including periodontitis. 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 For instance, Li et al., (2022) demonstrated the anti‐inflammatory role of lncRNA Nron in experimental periodontitis. Localized delivery of Nron in OC‐specific Nron KO mice inhibited alveolar bone resorption by promoting nuclear transport of NF‐kB repressing factor (NKRF), which significantly repressed Nfatc1 and induced resolution of inflammation. 199 Zhu et al. 200 reported that lncRNA APDC, an ortholog of human lncRNA ANRIL, plays an anti‐inflammatory role in periodontitis progression as observed by exacerbated bone loss and overt pro‐inflammatory cytokine expression in KO mice subjected to experimental periodontitis.
Studies from our laboratory have demonstrated the role of lncRNAs MALAT‐1, LIN01010 LRRC75A‐As1, and GAPLINC in periodontitis 201 , 202 , 203 , 204 ; Figure 7. In both periodontitis subjects and in vivo periodontitis murine model, MALAT1 was upregulated, while miR‐30b was downregulated in inflamed gingiva. MALAT1 sequesters miR‐30b by direct interaction to promote the inflammatory M1 Mφ phenotype as observed by higher M1 marker expression. Furthermore, MALAT1 knockdown attenuates bacterial phagocytosis, antigen processing, and inflammatory cytokine secretion. 202 Similarly, Valverde et al. 203 demonstrated a novel function of myeloid cell‐specific lncRNAs, LRRC75A‐As1 and GAPLINC. Both lncRNAs were observed to be upregulated in inflamed gingiva of periodontitis subjects. RNAi of LRRC75A‐As1 and GAPLINC resulted in a significant reduction of M1 Mφ expression markers without an impact on M2 markers. Thus, LINC01010 s can regulate key innate immune activities and polarization of Mφ, which are critical players in periodontitis progression and its resolution. 204
Together these findings indicate an important role of miRNAs and lncRNA‐miRNA regulatory networks in inflammatory pathways involved in periodontitis and could serve as novel therapeutic targets in periodontitis treatment or diagnosis. Future studies focusing on pre‐clinical models and clinical trials testing the validity of noncoding RNAs as a therapeutic option to treat or diagnose periodontitis are needed.
4.2.8. Antimicrobial photodynamic therapy (aPDT)
Antimicrobial photodynamic therapy (aPDT) is another alternative therapeutic approach based on the principle that visible light activates a non‐toxic molecule called photosensitizer, resulting in the generation of reactive oxygen species that non‐specifically kills bacteria via an oxidative burst. 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 However, studies have shown that aPDT is less aggressive in removing the commensal biofilms compared to regular systemic antibiotic therapy. 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 Multiple split‐mouth RCTs have reported that the use of aPDT as an adjunct to SRP, or when aPDT is applied multiple times in periodontitis patients, improves periodontal diagnostic indicators including a substantial decrease in BOP due to a reduction in periodontopathogens burden and associated inflammatory mediators (TNFα and IL1β) thus promoting restoration of periodontal homeostasis. 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214
4.3. Emerging treatment modalities for periodontitis
Alternative therapies using the above‐described anti‐resorptive and immunomodulatory drugs require tailored approaches to treat periodontitis. Novel therapeutic adjuncts to SRP include probiotics, statins/fibrates, vitamins, and omega‐3 fatty acids. 106 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239
As adjuncts to SRP, probiotics are new therapies targeting periodontopathogens aimed at the restoration of the healthy oral microbiome and thus a healthy periodontium. 106 , 215 , 216 , 217 , 218 , 219 , 220 The World Health Organization has defined probiotics as “living microorganisms that, when administered in appropriate amounts, confer health benefits to the host”. 215 Lactobacillus and Bifidobacterium are the most common probiotics used in periodontitis treatment. 218 , 219 Probiotics regulate the host's inflammatory response by secreting their own antimicrobial peptides or anti‐inflammatory molecules. In a recent systematic review, 10 RCTs assessed the impact of probiotics on clinical, microbial, and immunological outcomes. 217 , 218 , 219 , 220 , 221 As an adjunct to SRP, significant clinical benefits with greater PD reductions and CAL were noted after 12 months.
Studies indicate that periodontitis patients are more likely to have unfavorable serum lipid profiles and hence higher levels of inflammation, compared to periodontally healthy individuals. 222 , 223 , 224 The most commonly prescribed oral lipid‐lowering agents are statins and fibrates. Statins are used in patients with hypercholesterolemia while fibrates are useful for decreasing serum triglyceride levels. 222 , 223 , 224 , 225 Statins are inhibitors of HMG‐CoA reductase with anti‐inflammatory, anti‐coagulant, and antioxidant properties. 221 The anti‐inflammatory properties of statins have stimulated interest in the treatment of periodontitis. 222 Clinical investigations have reported that subgingival application of statin gels (atorvastatin, simvastatin, or rosuvastatin) as adjuncts to SRP significantly reduced levels of pro‐inflammatory mediators. Hence, an increase in anti‐inflammatory marker expression and a reduction in immune cells infiltration and bone resorption were noted. 222 Further, a retrospective cohort study of over 50 000 patients reported a 25.7% reduction of chronic periodontitis risk for >3 years in patients prescribed with a combinatorial treatment of statins and fibrates. 220
The role of nutrition in host‐modulation to prevent oral diseases and/or as an adjunct to periodontal therapy has gained attention. Recent studies have described the current understanding of the impact of impact of the diet supplemented with vitamins and other micro/macronutrients on preventing and treating periodontitis. 226 For instance, dietary nitrate and omega‐3 fatty acids have been proposed to suppress periodontal inflammation by different mechanisms. A recent RCT reported that subjects with generalized stage III or IV periodontitis receiving high doses of omega‐3 polyunsaturated fatty acids for 6 months resulted in an improved resolution of inflammation as noted by decreased PD and bleeding on probing (BOP). 227 , 228 , 229 , 230 , 231 However, the long‐term benefits of periodontal health remain unclear. In experimental periodontitis, animals fed with a dietary inorganic nitrate exhibited higher levels of NO, an osteoprotective and immunosuppressive free radical. This finding correlated with reduced pro‐inflammatory cytokine IL‐1β levels, endothelial inflammatory pathways, and tissue oxidative stress. Of note, higher anti‐inflammatory cytokine IL‐10 levels were also reported. 232
Resolvins are specialized SPM derived from omega‐3 fatty acids with anti‐inflammatory properties that suppress neutrophil infiltration and promote anti‐inflammatory cytokine production. 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 227 , 228 , 229 , 230 , 231 Two independent meta‐analyses of different clinical studies reported that omega‐3 fatty acid administration as an adjunct to SRP reduced PD and improved CAL in periodontitis subjects compared to placebo + SRP treatment. 227 , 228 , 229 , 230 Further, a recent RCT concluded that the intake of omega‐3 fatty acids as an adjunct to SRP reduced periodontitis progression by reducing periodontal pocket depths and increasing alveolar bone attachment. 225 Additional clinical studies are needed to confirm the functional efficacy of omega‐3 fatty acids as a therapeutic adjunct for the treatment of periodontitis.
Emerging evidence of the importance of nutritional modulation of periodontal inflammation was reported by the European Federation of Periodontology's European Workshop on Periodontology. 231 Vitamins that confer anti‐inflammatory and anti‐oxidative effects have been harnessed as a novel therapeutic approach to manage periodontitis. 227 , 228 , 229 , 230 , 231 In brief, a mice model of LIP treated with vitamin C reported inhibition of inflammation and reduced bone resorption associated with less OC differentiation and activation. 232 Vitamin B was administered daily in a rat model of LIP for 11 days resulting in bone tissue repair due to an increase in OB activity and reduced OC differentiation and activity. 233 Vitamin D exert immuno‐modulatory properties inhibiting the production of pro‐inflammatory cytokines and reducing the overactivation of immune cells such as CD4+, Th1, Th2, and Th17. 234 In addition, there is credible evidence that supports the role of Vitamin K, in promoting OB differentiation and reducing OB apoptosis thereby playing a key role in bone homeostasis. 235 , 236 , 237 , 238 , 239 Koshihara et al. 237 demonstrated the role of Vitamin K1 (phylloquinone) and K2 (menaquinone) in mesenchymal stem cells differentiation to OB by upregulating bone marker genes such as BMP‐2, and OPG and concomitant inhibition of RANKL or NF‐kB activation in OB. Yamaguchi and Weitzmann 238 analyzed the efficacy of Vitamin K1 and K2 in the treatment of osteoporosis in ovariectomized mice and concluded that Vitamin K2 had a greater effect compared to Vitamin K1 in preventing bone density loss in mice fed with a high‐fat diet compared to a normal diet. However, in a rat model of LIP, single treatments with either vitamin D3, vitamin K2 or a combination of both after SRP did not show any reduction in periodontitis‐associated pro‐inflammatory markers and OC activity‐associated bone resorption. 239 Together, these findings show that Vitamin K1 and K2 play a differential roles in alveolar bone homeostasis; albeit the results were not consistent and further studies are needed.
In summary, additional pre‐clinical testing and clinical trials using novel, non‐invasive therapeutic approaches are urgently needed. It will be important to take into consideration dosage, standardization, and patient compliance, among others.
5. CONCLUSIONS
Numerous molecular and cellular dynamics affect alveolar bone metabolism in periodontal health and disease. This comprehensive narrative review has detailed the current knowledge of impaired OB‐OC axis in periodontitis and the role of immune cells in controlling bone homeostasis and remodeling. The functional diversity of these factors may influence bone homeostasis during different stages of periodontitis as well as treatment outcomes. Therefore, a broader understanding of the cell‐specific dysregulated genes controlling bone remodeling beyond OB‐OC interactions will shed light on the underlying mechanisms and facilitate the development of novel therapeutic approaches. Strategies aimed at preventing the initiation of periodontitis or ameliorating the pathophysiologic events at the earlier stages of disease progression will minimize the need for surgical intervention. As stated earlier, adjunctive use of antibiotics after SRP has shown improved clinical outcomes but concerns regarding antibiotic resistance motivate the search for new, alternate drugs to treat periodontitis. A diverse spectrum of anti‐resorptive (bisphosphonates, OPG‐Fc, RANKL inhibitors, and strontium ranelate) and immunomodulatory agents (CTSK inhibitor, specialized pro‐resolving mediators, biological therapies, and noncoding RNAs) have shown promising outcomes in preventing or restoring the bone homeostasis in experimental periodontitis. The use of these candidate targets should be considered with caution only after comprehensive assessment in preclinical and Phase 1 clinical trials. For instance, the risk to ONJ development after bisphosphonate administration or DRONJ in the case of OPG‐Fc and RANKL inhibitors limits their clinical use. Clinical trials testing the efficacy of CTSK inhibitors (Balicatib and Odanacatib) and other experimental treatments (injections of small interfering RNA (siRNA), adeno‐associated virus (AAV), CTSK‐specific inhibitor, BML‐244, or CsinCPI‐2) have yielded promising results by reshaping bone metabolism or tissue immunity in periodontitis. However, adverse side effects (cerebrovascular accidents and cardiovascular events) have been reported.
Therapeutics targeting periodontitis, a host immune response‐associated disease, should be aimed at mitigating endogenous host mediators of inflammation and its resolution. Treatment with SPMs and biological therapies have shown promising outcomes by specifically targeting inflammatory genes associated with bone metabolism. Additionally, specific noncoding RNAs have been identified with significant roles in regulating immunity and osteoclastic activity. Further studies and clinical trials are needed to validate their use as therapeutic agents. Other alternative emerging treatment options such as probiotics, statins, vitamins, and omega‐3 fatty acids have gained attention due to their anti‐inflammatory and anti‐resorptive properties with limited or no side effects. However, the effective doses of these natural antimicrobial compounds need to be determined and their antimicrobial mechanisms should be further delineated before their use as therapeutic adjuncts. Novel microbiome‐based approaches have demonstrated exciting outcomes in treating experimental periodontitis; however, further research is needed to improve their efficacy and to understand their long‐term effects. Interaction of candidate drug targets with modifiable factors including smoking, nutrition, and oral hygiene should also be investigated to assess their therapeutic efficacy.
Current treatment strategies remain moderately effective in delaying disease progression and regenerating the periodontium and are expensive. There is a need for the development of more cost‐effective and innovative therapeutic approaches that can predict the onset of disease and arrest its progression. Specifically, personalized treatment plans based on precision medicine tools will become mainstream. To monitor the progression of periodontitis and post‐treatment outcomes, identification, and validation of specific and sensitive biomarkers will provide a better assessment of periodontal health that may help clinicians personalize treatment strategies for each patient. While the significance of personalized medicine based on prognostic biomarkers of periodontitis is immense, it remains largely understudied. Changes in cellular and molecular mediators precede the clinical manifestation of periodontitis and its resolution. Therefore, novel approaches aimed at harnessing the potential of tissue‐relevant biological changes will help us understand the disease and advance therapeutic intervention. This can be aided by comprehensive multi‐omic approaches to comprehensively screen host and pathogen‐derived molecules to develop unique markers of disease severity and response to periodontal therapies. Large, multicenter‐randomized controlled trials involving different genders, races, and age groups should be undertaken to evaluate the clinical value of predictive markers of periodontitis to personally prevent or treat the disease.
FUNDING INFORMATION
NIDCR/NIH; contract graft numbers: R01DE027980 (ARN), R56DE033249 (ARN), DE021052 (AG), DE031737 (AG), and DE021052 (SN); NEI/NIH contract grant number R01EY033622 (ARN); The Brodie Endowment Fund (AG).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
Valverde A, George A, Nares S, Naqvi AR. Emerging therapeutic strategies targeting bone signaling pathways in periodontitis. J Periodont Res. 2025;60:101‐120. doi: 10.1111/jre.13326
Contributor Information
Araceli Valverde, Email: avalverd@uic.edu.
Afsar R. Naqvi, Email: afsarraz@uic.edu.
DATA AVAILABILITY STATEMENT
No original data were used in this manuscript.
REFERENCES
- 1. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 2. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. 2006;40(1):11‐28. [DOI] [PubMed] [Google Scholar]
- 3. Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020;83(1):26‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. 2020;83(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 5. Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: basic biological considerations. Periodontol 2000. 2021;87(1):143‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen LM, Chon JJ, Kim EE, Cheng JC, Ebersole JL. Biological aging and periodontal disease: analysis of NHANES (2001–2002). JDR Clin Trans Res. 2022;7(2):145‐153. [DOI] [PubMed] [Google Scholar]
- 7. Leung TJT, Nijland N, Gerdes VEA, Loos BG. Prevalence of periodontal disease among patients at the outpatient clinic of internal medicine in an academic hospital in The Netherlands: a cross‐sectional pilot study. J Clin Med. 2022;11(20):6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249‐260. [DOI] [PubMed] [Google Scholar]
- 9. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martínez M, Postolache TT, García‐Bueno B, et al. The role of the oral microbiota related to periodontal diseases in anxiety, mood and trauma‐ and stress‐related disorders. Front Psych. 2022;27(12):814177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome‐host interactions. Comput Struct Biotechnol J. 2021;27(19):1335‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guentsch A, Puklo M, Preshaw PM, et al. Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans . J Periodontal Res. 2009;44(3):368‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grenier D, Tanabe S. Porphyromonas gingivalis gingipains trigger a proinflammatory response in human monocyte‐derived macrophages through the p38α mitogen‐activated protein kinase signal transduction pathway. Toxins (Basel). 2010;2(3):341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schröder A, Wagner K, Cieplik F, Spanier G, Proff P, Kirschneck C. Impact of phosphorylation of heat shock protein 27 on the expression profile of periodontal ligament fibroblasts during mechanical strain. J Orofac Orthop. 2023;84(Suppl 2):143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaidi M, Alam ASMT, Shankar VS, et al. Cellular biology of bone resorption. Biol Rev Camb Philos Soc. 1993;68:197‐264. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz Z, Goultschin J, Dean DD, Boyan BD. Mechanisms of alveolar bone destruction in periodontitis. Periodontol 2000. 1997;14(1):158‐172. [DOI] [PubMed] [Google Scholar]
- 17. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuo K, Irie N. Osteoclast‐osteoblast communication. Arch Biochem Biophys. 2008;473:201‐209. [DOI] [PubMed] [Google Scholar]
- 19. Tadjoedin FM, Fitri AH, Kuswandani SO, Sulijaya B, Soeroso Y. The correlation between age and periodontal diseases. J Int Dent Med Res. 2017;10(2):327‐332. [Google Scholar]
- 20. Tan J, Dai A, Pan L, et al. Inflamm‐aging‐related cytokines of IL‐17 and IFN‐γ accelerate osteoclastogenesis and periodontal destruction. J Immunol Res. 2021;2021:9919024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martínez‐García M, Hernández‐Lemus E. Periodontal inflammation and systemic diseases: an overview. Front Physiol. 2021;27(12):709438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pai SI, Matheus HR, Guastaldi FPS. Effects of periodontitis on cancer outcomes in the era of immunotherapy. Lancet Healthy Longev. 2023;4(4):e166‐e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Păunică I, Giurgiu M, Dumitriu AS, et al. The bidirectional relationship between periodontal disease and diabetes mellitus‐a review. Diagnostics (Basel). 2023;13(4):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast‐osteoclast interactions. Connect Tissue Res. 2018;59(2):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamma R, Zallone A. Osteoblast and osteoclast crosstalks: from OAF to Ephrin. Inflamm Allergy Drug Targets. 2012;11:196‐200. [DOI] [PubMed] [Google Scholar]
- 26. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast‐osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23(3):329‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61‐87. [DOI] [PubMed] [Google Scholar]
- 29. Papapanou PN. Risk assessments in the diagnosis and treatment of periodontal diseases. J Dent Educ. 1998;62:822‐839. [PubMed] [Google Scholar]
- 30. Pihlstrom BL. Periodontal risk assessment, diagnosis, and treatment planning. Periodontol 2000. 2001;25:37‐58. [DOI] [PubMed] [Google Scholar]
- 31. Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host‐modulation therapy. Periodontol 2000. 2020;84(1):14‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwon T, Lamster IB, Levin L. Current concepts in the management of periodontitis. Int Dent J. 2021;71(6):462‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dutzan N, Konkel JE, Greenwell‐Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark D, Radaic A, Kapila Y. Cellular mechanism of inflammaging and periodontal disease. Front Dent Med. 2022;3:844865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vitkov L, Muñoz LE, Schoen J, et al. Neutrophils orchestrate the periodontal pocket. Front Immunol. 2021;12:788766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontol 2000. 2014;64:111‐126. [DOI] [PubMed] [Google Scholar]
- 37. Loos BG, Roos MT, Schellekens PT, van der Velden U, Miedema F. Lymphocyte numbers and function in relation to periodontitis and smoking. J Periodontol. 2004;75:557‐564. [DOI] [PubMed] [Google Scholar]
- 38. Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth‐supporting tissues. J Clin Med. 2019;8(8):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Ling J, Jiang Q. Inflammasomes in alveolar bone loss. Front Immunol. 2021;12:691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Slots J. Focal infection of periodontal origin. Periodontol 2000. 2019;79(1):233‐235. [DOI] [PubMed] [Google Scholar]
- 41. Colombo APV, do Souto RM, da Silva‐Boghossian CM, et al. Microbiology of oral biofilm‐dependent diseases: have we made significant progress to understand and treat these diseases? Curr Oral Health Rep. 2015;2:37‐47. [Google Scholar]
- 42. McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J Periodontol. 2002;73(11):1377‐1391. [DOI] [PubMed] [Google Scholar]
- 43. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64(1):57‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque‐induced gingivitis: case definition and diagnostic considerations. J Clin Periodontol. 2018;45(Suppl 20):S44‐S67. [DOI] [PubMed] [Google Scholar]
- 45. Gursoy UK, Könönen E, Luukkonen N, Uitto VJ. Human neutrophil defensins and their effect on epithelial cells. J Periodontol. 2013;84:126‐133. [DOI] [PubMed] [Google Scholar]
- 46. Benakanakere M, Kinane DF. Innate cellular responses to the periodontal biofilm. Front Oral Biol. 2012;15:41‐55. [DOI] [PubMed] [Google Scholar]
- 47. Miralda I, Uriarte SM. Periodontal pathogens' strategies disarm neutrophils to promote dysregulated inflammation. Mol Oral Microbiol. 2021;36(2):103‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu J, Du X, Chen J, Hu L, Chen L. The induction expression of human β‐defensins in gingival epithelial cells and fibroblasts. Arch Oral Biol. 2013;58:1415‐1421. [DOI] [PubMed] [Google Scholar]
- 49. Fine N, Hassanpour S, Borenstein A, et al. Distinct oral neutrophil subsets define health and periodontal disease states. J Dent Res. 2016;95(8):931‐938. [DOI] [PubMed] [Google Scholar]
- 50. Rijkschroeff P, Loos BG, Nicu EA. Oral polymorphonuclear neutrophil contributes to oral health. Curr Oral Health Rep. 2018;5(4):211‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44(5):664‐672. [DOI] [PubMed] [Google Scholar]
- 52. Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Neutrophil fate in gingival crevicular fluid. Ultrastruct Pathol. 2010;34(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 53. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 54. Song B, Zhang YL, Chen LJ, et al. The role of toll‐like receptors in periodontitis. Oral Dis. 2017;23:168‐180. [DOI] [PubMed] [Google Scholar]
- 55. Fteita D, Könönen E, Gürsoy M, Ma X, Sintim HO, Gürsoy UK. Quorum sensing molecules regulate epithelial cytokine response and biofilm‐related virulence of three prevotella species. Anaerobe. 2018;54:128‐135. [DOI] [PubMed] [Google Scholar]
- 56. Hiroshima Y, Bando M, Kataoka M, et al. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin‐1. Arch Oral Biol. 2011;56:761‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143‐156. [DOI] [PubMed] [Google Scholar]
- 58. Wilensky A, Segev H, Mizraji G, et al. Dendritic cells and their role in periodontal disease. Oral Dis. 2014;20(2):119‐126. [DOI] [PubMed] [Google Scholar]
- 59. Song L, Dong G, Guo L, Graves DT. The function of dendritic cells in modulating the host response. Mol Oral Microbiol. 2018;33:13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL‐17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;41:541‐549. [DOI] [PubMed] [Google Scholar]
- 61. Leija‐Montoya AG, González‐Ramírez J, Serafín‐Higuera I, Sandoval‐Basilio J, Isiordia‐Espinoza M, Serafín‐Higuera N. Emerging avenues linking myeloid‐derived suppressor cells to periodontal disease. Int Rev Cell Mol Biol. 2023;375:165‐189. [DOI] [PubMed] [Google Scholar]
- 62. Cai X, Li Z, Zhao Y, et al. Enhanced dual function of osteoclast precursors following calvarial Porphyromonas gingivalis infection. J Periodontal Res. 2020;55(3):410‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629‐648. [DOI] [PubMed] [Google Scholar]
- 64. Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27‐38. [DOI] [PubMed] [Google Scholar]
- 65. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349‐355. [DOI] [PubMed] [Google Scholar]
- 66. Jiang H, Sodek J, Karsenty G, Thomas H, Ranly D, Chen J. Expression of core binding factor Osf2/Cbfa‐1 and bone sialoprotein in tooth development. Mech Dev. 1999;81(1–2):169‐173. [DOI] [PubMed] [Google Scholar]
- 67. Kim JY, Kim BI, Jue SS, Park JH, Shin JW. Localization of osteopontin and osterix in periodontal tissue during orthodontic tooth movement in rats. Angle Orthod. 2012;82(1):107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao Y, Wang C, Li S, et al. Expression of osterix in mechanical stress‐induced osteogenic differentiation of periodontal ligament cells in vitro. Eur J Oral Sci. 2008;116(3):199‐206. [DOI] [PubMed] [Google Scholar]
- 69. Huang X, Su X, Ma Q, et al. FoxO1 agonists promote bone regeneration in periodontitis by protecting the osteogenesis of periodontal ligament stem cells. Stem Cells Dev. 2023;32(15–16):491‐503. [DOI] [PubMed] [Google Scholar]
- 70. Nakashima T, Takayanagi H. Osteoclasts, and the immune system. J Bone Miner Metab. 2009;27:519‐529. [DOI] [PubMed] [Google Scholar]
- 71. Zhang ZH, Jia XY, Fang JY, et al. Reduction of SOST gene promotes bone formation through the Wnt/β‐catenin signaling pathway and compensates particle‐induced osteolysis. J Cell Mol Med. 2020;24:4233‐4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang F, Huang D, Xu L, et al. Wnt antagonist secreted frizzled‐related protein I (sFRP1) may be involved in the osteogenic differentiation of periodontal ligament cells in chronic apical periodontitis. Int Endod J. 2021;54(5):768‐779. [DOI] [PubMed] [Google Scholar]
- 73. Alloisio G, Ciaccio C, Fasciglione GF, et al. Effects of extracellular osteoanabolic agents on the endogenous response of osteoblastic cells. Cells. 2021;10(9):2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Corral DA, Amling M, Priemel M, et al. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci USA. 1998;95(23):13835‐13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zheng H, Yu X, Collin‐Osdoby P, Osdoby P. RANKL stimulates inducible nitric‐oxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKL‐induced interferon‐beta via NF‐kappaB that restrains osteoclastogenesis and bone resorption. J Biol Chem. 2006;281(23):15809‐15820. [DOI] [PubMed] [Google Scholar]
- 76. Tsutsumi R, Xie C, Wei X, et al. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res. 2009;24(10):1753‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guder C, Gravius S, Burger C, Wirtz DC, Schildberg FA. Osteoimmunology: a current update of the interplay between bone and the immune system. Front Immunol. 2020;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thomas S, Jaganathan BG. Signaling network regulating osteogenesis in mesenchymal stem cells. J Cell Commun Signal. 2022;16(1):47‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. AlQranei MS, Chellaiah MA. Osteoclastogenesis in periodontal diseases: possible mediators and mechanisms. J Oral Biosci. 2020;62(2):123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 81. Bruzzaniti A, Baron R. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord. 2006;7(1–2):123‐139. [DOI] [PubMed] [Google Scholar]
- 82. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504‐1508. [DOI] [PubMed] [Google Scholar]
- 83. Bar‐Shavit Z. The osteoclast: a multinucleated, hematopoietic origin, bone‐resorbing osteoimmune cell. J Cell Biochem. 2007;102(5):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 84. Quinn JMW, Neale S, Fujikawa Y, McGee JD, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62(6):527‐531. [DOI] [PubMed] [Google Scholar]
- 85. Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597‐3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Oates TW, Cochran DL. Bone cell interactions and regulation by inflammatory mediators. Curr Opin Periodontol. 1996;3:34‐44. [PubMed] [Google Scholar]
- 89. Lu EM. The role of vitamin D in periodontal health and disease. J Periodontal Res. 2023;58(2):213‐224. [DOI] [PubMed] [Google Scholar]
- 90. MacDonald BR. Parathyroid hormone, prostaglandins and bone resorption. World Rev Nutr Diet. 1986;47:163‐201. [DOI] [PubMed] [Google Scholar]
- 91. Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85(6):508‐518. [DOI] [PubMed] [Google Scholar]
- 92. Fung KY, Louis C, Metcalfe RD, et al. Emerging roles for IL‐11 in inflammatory diseases. Cytokine. 2022;149:155750. [DOI] [PubMed] [Google Scholar]
- 93. Tonna S, Takyar FM, Vrahnas C, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB J. 2014;28:4482‐4496. [DOI] [PubMed] [Google Scholar]
- 94. Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917‐924. [DOI] [PubMed] [Google Scholar]
- 95. Xu R. Semaphorin 3A: a new player in bone remodeling. Cell Adh Migr. 2014;8:5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989;224(2):317‐324. [DOI] [PubMed] [Google Scholar]
- 97. Bord S, Horner A, Hembry RM, Reynolds A, Compston JE. Production of collagenase by human osteoblasts and osteoclasts in vivo. Bone. 1996;19(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 98. Chambers TJ. The pathobiology of the osteoclast. J Clin Pathol. 1985;38(3):241‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sasaki T, Garant PR. Structure and organization of odontoblasts. Anat Rec. 1996;245(2):235‐249. [DOI] [PubMed] [Google Scholar]
- 100. Parameter on chronic periodontitis with advanced loss of periodontal support. American Academy of periodontology. J Periodontol. 2000;71(5 Suppl):856‐858. [DOI] [PubMed] [Google Scholar]
- 101. American Academy of Periodontology . Comprehensive periodontal therapy: a statement by the American Academy of Periodontology102*. J Periodontol. 2011;82(7):943‐949. [DOI] [PubMed] [Google Scholar]
- 102. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017;2(4):224‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dye BA. Global periodontal disease epidemiology. Periodontol 2000. 2012;58:5810‐5825. [DOI] [PubMed] [Google Scholar]
- 104. Albandar JM. Epidemiology and risk factors of periodontal diseases. Dent Clin N Am. 2005;49:517‐532. [DOI] [PubMed] [Google Scholar]
- 105. Nogueira AH, Furlaneto F, Gaio EJ, et al. New tendencies in non‐surgical periodontal therapy. Braz Oral Res. 2021;35(supp2):e095. [DOI] [PubMed] [Google Scholar]
- 106. Haque MM, Yerex K, Kelekis‐Cholakis A, Duan K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health. 2022;22(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pavanelli ALR, de Menezes BS, Pereira EBB, de Souza Morais FA, Cirelli JA, de Molon RS. Pharmacological therapies for the management of inflammatory bone resorption in periodontal disease: a review of preclinical studies. Biomed Res Int. 2022;2022:5832009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kwon T, Wang JCW, Levin L. Home care is therapeutic. Should we use the term home‐care therapy instead of instructions? Oral Health Prev Dent. 2020;18:397‐398. [DOI] [PubMed] [Google Scholar]
- 109. Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71(4):521‐532. [DOI] [PubMed] [Google Scholar]
- 110. Kwon T, Salem DM, Levin L. Nonsurgical periodontal therapy based on the principles of cause‐related therapy: rationale and case series. Quintessence Int. 2019;50(5):370‐376. [DOI] [PubMed] [Google Scholar]
- 111. Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double‐blind placebo‐controlled study. J Clin Periodontol. 2001;28(4):296‐305. [DOI] [PubMed] [Google Scholar]
- 112. Liaw A, Miller C, Nimmo A. Comparing the periodontal tissue response to non‐surgical scaling and root planing alone, adjunctive azithromycin, or adjunctive amoxicillin plus metronidazole in generalized chronic moderate‐to‐severe periodontitis: a preliminary randomized controlled trial. Aust Dent J. 2019;64(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 113. McGowan K, McGowan T, Ivanovski S. Optimal dose and duration of amoxicillin‐plus‐metronidazole as an adjunct to non‐surgical periodontal therapy: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Periodontol. 2018;45(1):56‐67. [DOI] [PubMed] [Google Scholar]
- 114. Carnevale G, Kaldahl WB. Osseous resective surgery. Periodontol 2000. 2000;22:59‐87. [DOI] [PubMed] [Google Scholar]
- 115. Villar CC, Cochran DL. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin N Am. 2010;54(1):73‐92. [DOI] [PubMed] [Google Scholar]
- 116. Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen‐containing bisphosphonates. J Dent Res. 2007;86(11):1022‐1033. [DOI] [PubMed] [Google Scholar]
- 117. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen‐containing bisphosphonates inhibit the mevalonate pathway and prevent post‐translational prenylation of GTP‐binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581‐589. [DOI] [PubMed] [Google Scholar]
- 118. Muniz FWMG, Silva BFD, Goulart CR, Silveira TMD, Martins TM. Effect of adjuvant bisphosphonates on treatment of periodontitis: systematic review with meta‐analyses. J Oral Biol Craniofac Res. 2021;11(2):158‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ruggiero SL, Mehrotra B. Bisphosphonate‐related osteonecrosis of the jaw: diagnosis, prevention, and management. Annu Rev Med. 2009;60:85‐96. [DOI] [PubMed] [Google Scholar]
- 120. Moreira MM, Bradaschia‐Correa V, Marques ND, Ferreira LB, Arana‐Chavez VE. Ultrastructural and immunohistochemical study of the effect of sodium alendronate in the progression of experimental periodontitis in rats. Microsc Res Tech. 2014;77(11):902‐909. [DOI] [PubMed] [Google Scholar]
- 121. De Almeida J, Ervolino E, Bonfietti LH, et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol. 2015;86(10):1166‐1175. [DOI] [PubMed] [Google Scholar]
- 122. Rocha M, Nava LE, de la Torre CV, Sánchez‐Marín F, Garay‐Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo‐controlled trial. J Periodontol. 2001;72:204‐209. [DOI] [PubMed] [Google Scholar]
- 123. Rocha ML, Malacara JM, Sánchez‐Marin FJ, de la Torre CJV, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo‐controlled trial. J Periodontol. 2004;75:1579‐1585. [DOI] [PubMed] [Google Scholar]
- 124. Lane N, Armitage GC, Loomer P, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12‐month, randomized, placebo‐controlled study. J Periodontol. 2005;76(7):1113‐1122. [DOI] [PubMed] [Google Scholar]
- 125. Graziani F, Cei S, Guerrero A, et al. Lack of short‐term adjunctive effect of systemic neridronate in non‐surgical periodontal therapy of advanced generalized chronic periodontitis: an open label‐randomized clinical trial. J Clin Periodontol. 2009;36(5):419‐427. [DOI] [PubMed] [Google Scholar]
- 126. Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83:19‐26. [DOI] [PubMed] [Google Scholar]
- 127. Pradeep AR, Sharma A, Rao NS, Bajaj P, Naik SB, Kumari M. Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double‐masked controlled clinical trial. J Periodontol. 2012;83:1322‐1328. [DOI] [PubMed] [Google Scholar]
- 128. Alwithanani N. Role of bisphosphonates in periodontal disease: systematic review. J Pharm Bioallied Sci. 2023;15(Suppl 1):S46‐S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Long‐term safety of bisphosphonates. J Clin Endocrinol Metabol. 2005;90(3):1897‐1899. [DOI] [PubMed] [Google Scholar]
- 130. Teng YT, Nguyen H, Gao X, et al. Functional human T‐cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106(6):R59‐R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66(12):1139‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jiang L, Dong J, Wei J, Liu L. Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid‐induced osteoporosis: a systematic review and meta‐analysis. BMC Musculoskelet Disord. 2022;23(1):1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677‐692. [DOI] [PubMed] [Google Scholar]
- 134. de Molon RS, Cheong S, Bezouglaia O, et al. Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone. 2014;68:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Okuma S, Matsuda Y, Nariai Y, Karino M, Suzuki R, Kanno T. A retrospective observational study of risk factors for denosumab‐related osteonecrosis of the jaw in patients with bone metastases from solid cancers. Cancers (Basel). 2020;12(5):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rodríguez J, Escudero ND, Mandalunis PM. Effect of strontium ranelate on bone remodeling. Acta Odontol Latinoam. 2012;25(2):208‐213. [PubMed] [Google Scholar]
- 137. Marie PJ. Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone. 2006;38(2 Suppl 1):S10‐S14. [DOI] [PubMed] [Google Scholar]
- 138. Ammann P, Badoud I, Barraud S, Dayer R, Rizzoli R. Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res. 2007;22(9):1419‐1425. [DOI] [PubMed] [Google Scholar]
- 139. Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium‐sensing receptor (CaR) is involved in strontium ranelate‐induced osteoblast proliferation. Biochem Pharmacol. 2007;74(3):438‐447. [DOI] [PubMed] [Google Scholar]
- 140. Karakan NC, Akpınar A, Göze F, Poyraz Ö. Investigating the effects of systemically administered strontium Ranelate on alveolar bone loss histomorphometrically and histopathologically on experimental periodontitis in rats. J Periodontol. 2017;88(2):e24‐e31. [DOI] [PubMed] [Google Scholar]
- 141. Marie PJ, Hott M, Modrowski D, De Pollak C, et al. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen‐deficient rats. J Bone Miner Res. 1993;8(5):607‐615. [DOI] [PubMed] [Google Scholar]
- 142. Souza RB, Gomes FIF, Pereira KMA, et al. Strontium Ranelate elevates expression of Heme Oxygenase‐1 and decreases alveolar bone loss in rats. J Oral Maxillofac Res. 2018;9(4):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang B, Pang X, Li Z, Chen Z, Wang Y. Immunomodulation in the treatment of periodontitis: progress and perspectives. Front Immunol. 2021;12:781378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273(48):32347‐32352. [DOI] [PubMed] [Google Scholar]
- 145. Qian D, He L, Zhang Q, et al. Cathepsin K: a versatile potential biomarker and therapeutic target for various cancers. Curr Oncol. 2022;29(8):5963‐5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stoch SA, Wagner JA. Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther. 2008;83(1):172‐176. [DOI] [PubMed] [Google Scholar]
- 147. Drake MT, Clarke BL, Oursler MJ, Khosla S. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. 2017;38(4):325‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. McClung MR, O'Donoghue ML, Papapoulos SE, et al. Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double‐blind, placebo‐controlled trial and LOFT extension study. Lancet Diabetes Endocrinol. 2019;7(12):899‐911. [DOI] [PubMed] [Google Scholar]
- 149. Han J, Wei L, Xu W, et al. CTSK inhibitor exert its anti‐obesity effects through regulating adipocyte differentiation in high‐fat diet induced obese mice. Endocr J. 2015;62(4):309‐317. [DOI] [PubMed] [Google Scholar]
- 150. Hao L, Chen J, Zhu Z, et al. Odanacatib, a Cathepsin K‐specific inhibitor, inhibits inflammation and bone loss caused by periodontal diseases. J Periodontol. 2015;86(8):972‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen W, Gao B, Hao L, et al. The silencing of cathepsin K used in gene therapy for periodontal disease reveals the role of cathepsin K in chronic infection and inflammation. J Periodontal Res. 2016;51(5):647‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yue Y, Yin W, Yang Q, et al. Inhibition of Cathepsin K alleviates autophagy‐related inflammation in periodontitis‐aggravating arthritis. Infect Immun. 2020;88(12):e00498‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Falgueyret JP, Desmarais S, Oballa R, et al. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off‐target cathepsins and reduced functional selectivity. J Med Chem. 2005;48(24):7535‐7543. [DOI] [PubMed] [Google Scholar]
- 154. Jerome C, Missbach M, Gamse R. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int. 2011;22(12):3001‐3011. [DOI] [PubMed] [Google Scholar]
- 155. Adami SSJ, Hala T, Brown JP, et al. Effect of one year treatment with the cathepsin‐K inhibitor, balicatib, on bone mineral density (BMD) in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Res. 2006;21(Suppl 1):S24. Abstract 1085. [Google Scholar]
- 156. Lewiecki EM. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs. 2009;12(12):799‐809. [PubMed] [Google Scholar]
- 157. McClung MR, Papapoulos S, Saag KG, et al. Odanacatib efficacy and safety in postmenopausal women with osteoporosis: 5‐year data from the extension of the phase 3 Long‐Term Odanacatib Fracture Trial (LOFT). American Society for Bone and Mineral Research Annual Meeting, Atlanta, Georgia; 2016.
- 158. Papapoulos S, McClung MR, Langdahl B, et al. Safety of odanacatib in postmenopausal women with osteoporosis: 5‐year data from the extension of the phase 3 Long‐Term Odanacatib Fracture Trial (LOFT). American Society for Bone and Mineral Research Annual Meeting, Atlanta, Georgia; 2016.
- 159. O'Donoghue M, Cavallari I, Bonaca M, et al. The Long‐Term Odanacatib Fracture Trial (LOFT): cardiovascular safety results. American Society for Bone and Mineral Research Annual Meeting, Atlanta, Georgia; 2016.
- 160. Pan W, Yin W, Yang L, et al. Inhibition of Ctsk alleviates periodontitis and comorbid rheumatoid arthritis via downregulation of the TLR9 signalling pathway. J Clin Periodontol. 2019;46(3):286‐296. [DOI] [PubMed] [Google Scholar]
- 161. Da Ponte Leguizamón N, de Molon RS, Coletto‐Nunes G, et al. Phytocystatin CsinCPI‐2 reduces osteoclastogenesis and alveolar bone loss. J Dent Res. 2022;101(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 162. Brömme D, Lecaille F. Cathepsin K inhibitors for osteoporosis and potential off‐target effects. Expert Opin Investig Drugs. 2009;18(5):585‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti‐inflammatory and pro‐resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host modulation and treatment of periodontal disease. J Dent Res. 2021;100(8):798‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. de Molon RS, Thurlings RM, Walgreen B, et al. Systemic Resolvin E1 (RvE1) treatment does not ameliorate the severity of collagen‐induced arthritis (CIA) in mice: a randomized, prospective, and controlled proof of concept study. Mediators Inflamm. 2019;2019:5689465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL‐37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25(5):1697‐1705. [DOI] [PubMed] [Google Scholar]
- 168. Gao L, Faibish D, Fredman G, et al. Resolvin E1 and chemokine‐like receptor 1 mediate bone preservation. J Immunol. 2013;190(2):689‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lee CT, Teles R, Kantarci A, et al. Resolvin E1 reverses experimental periodontitis and dysbiosis. J Immunol. 2016;197(7):2796‐2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast‐ mediated bone destruction in periodontitis. FASEB J. 2006;20(2):401‐403. [DOI] [PubMed] [Google Scholar]
- 171. Hasturk H, Kantarci A, Goguet‐Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179(10):7021‐7029. [DOI] [PubMed] [Google Scholar]
- 172. Hasturk H, Schulte F, Martins M, et al. Safety and preliminary efficacy of a novel host‐modulatory therapy for reducing gingival inflammation. Front Immunol. 2021;12:704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mizraji G, Heyman O, Van Dyke TE, Wilensky A. Resolvin D2 restrains Th1 immunity and prevents alveolar bone loss in murine periodontitis. Front Immunol. 2018;9:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Alshibani N. Resolvins as a treatment modality in experimental periodontitis: a systematic review of preclinical studies. Cureus. 2022;14(1):e21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Apolinário Vieira GH, Aparecida Rivas AC, Figueiredo Costa K, et al. Specific inhibition of IL‐6 receptor attenuates inflammatory bone loss in experimental periodontitis. J Periodontol. 2021;92(10):1460‐1469. [DOI] [PubMed] [Google Scholar]
- 176. Grauballe MB, Østergaard JA, Schou S, Flyvbjerg A, Holmstrup P. Effects of TNF‐α blocking on experimental periodontitis and type 2 diabetes in obese diabetic Zucker rats. J Clin Periodontol. 2015;42(9):807‐816. [DOI] [PubMed] [Google Scholar]
- 177. Grauballe MB, Belstrøm D, Østergaard JA, et al. Ligature‐associated bacterial profiles are linked to type 2 diabetes mellitus in a rat model and influenced by antibody treatment against TNF‐α or RAGE. Clin Exp Dent Res. 2017;3(1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Oates TW, Graves DT, Cochran DL. Clinical, radiographic and biochemical assessment of IL‐1/TNF‐alpha antagonist inhibition of bone loss in experimental periodontitis. J Clin Periodontol. 2002;29(2):137‐143. [DOI] [PubMed] [Google Scholar]
- 179. Queiroz‐Junior CM, Bessoni RL, Costa VV, Souza DG, Teixeira MM, Silva TA. Preventive and therapeutic anti‐TNF‐α therapy with pentoxifylline decreases arthritis and the associated periodontal co‐morbidity in mice. Life Sci. 2013;93(9–11):423‐428. [DOI] [PubMed] [Google Scholar]
- 180. Santonocito S, Polizzi A, Palazzo G, Isola G. The emerging role of microRNA in periodontitis: pathophysiology, clinical potential and future molecular perspectives. Int J Mol Sci. 2021;22(11):5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Luan X, Zhou X, Naqvi A, et al. MicroRNAs and immunity in periodontal health and disease. Int J Oral Sci. 2018;10(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Luan X, Zhou X, Trombetta‐eSilva J, et al. MicroRNAs and periodontal homeostasis. J Dent Res. 2017;96(5):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Arif KMT, Elliott EK, Haupt LM, Griffiths LR. Regulatory mechanisms of epigenetic miRNA relationships in human cancer and potential as therapeutic targets. Cancers (Basel). 2020;12(10):2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sattari M, Taheri RA, ArefNezhad R, Motedayyen H. The expression levels of MicroRNA‐146a, RANKL and OPG after non‐surgical periodontal treatment. BMC Oral Health. 2021;21(1):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Motedayyen H, Ghotloo S, Saffari M, Sattari M, Amid R. Evaluation of MicroRNA‐146a and its targets in gingival tissues of patients with chronic periodontitis. J Periodontol. 2015;86(12):1380‐1385. [DOI] [PubMed] [Google Scholar]
- 186. Gao Y, Wang B, Shen C, Xin W. Overexpression of miR‐146a blocks the effect of LPS on RANKL‐induced osteoclast differentiation. Mol Med Rep. 2018;18(6):5481‐5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ghotloo S, Motedayyen H, Amani D, Saffari M, Sattari M. Assessment of microRNA‐146a in generalized aggressive periodontitis and its association with disease severity. J Periodontal Res. 2019;54:27‐32. [DOI] [PubMed] [Google Scholar]
- 188. Zhou W, Su L, Duan X, et al. MicroRNA‐21 down‐regulates inflammation and inhibits periodontitis. Mol Immunol. 2018;101:608‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Akkouch A, Zhu M, Romero‐Bustillos M, et al. MicroRNA‐200c attenuates periodontitis by modulating proinflammatory and osteoclastogenic mediators. Stem Cells Dev. 2019;28:1026‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wendlandt EB, Graff JW, Gioannini TL, McCaffrey AP, Wilson ME. The role of microRNAs miR‐200b and miR‐200c in TLR4 signaling and NF‐κB activation. Innate Immun. 2012;18:846‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Aziz F. The emerging role of miR‐223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol. 2016;303:1‐6. [DOI] [PubMed] [Google Scholar]
- 192. Pettiette MT, Zhang S, Moretti AJ, Kim SJ, Naqvi AR, Nares S. MicroRNA expression profiles in external cervical resorption. J Endod. 2019;45(9):1106‐1113.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Naqvi AR, Brambila MF, Martínez G, Chapa G, Nares S. Dysregulation of human miRNAs and increased prevalence of HHV miRNAs in obese periodontitis subjects. J Clin Periodontol. 2019;46(1):51‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Valverde A, Nares S, Naqvi AR. Impaired cell migration and structural defects in myeloid cells overexpressing miR‐30b and miR‐142‐3p. Biochim Biophys Acta Gene Regul Mech. 2020;1863(11):194628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Uttamani JR, Naqvi AR, Estepa AMV, et al. Downregulation of miRNA‐26 in chronic periodontitis interferes with innate immune responses and cell migration by targeting phospholipase C beta 1. J Clin Periodontol. 2023;50(1):102‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1‐46. [DOI] [PubMed] [Google Scholar]
- 197. Jathar S, Kumar V, Srivastava J, Tripathi V. Technological developments in lncRNA biology. Adv Exp Med Biol. 2017;1008:283‐323. [DOI] [PubMed] [Google Scholar]
- 198. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354‐361. [DOI] [PubMed] [Google Scholar]
- 199. Li J, Jin F, Cai M, Lin T, Wang X, Sun Y. LncRNA Nron inhibits bone resorption in periodontitis. J Dent Res. 2022;101(2):187‐195. [DOI] [PubMed] [Google Scholar]
- 200. Zhu ZX, Liu Y, Wang J, et al. A novel lncRNA‐mediated epigenetic regulatory mechanism in periodontitis. Int J Biol Sci. 2023;19(16):5187‐5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ahmad I, Valverde A, Naqvi RA, Naqvi AR. Long noncoding RNAs RN7SK and GAS5 regulate macrophage polarization and innate immune responses. Front Immunol. 2021;11:604981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ahmad I, Naqvi RA, Valverde A, Naqvi AR. LncRNA MALAT1/microRNA‐30b axis regulates macrophage polarization and function. Front Immunol. 2023;14:1214810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Valverde A, Naqvi RA, Naqvi AR. Macrophage‐enriched novel functional long noncoding RNAs LRRC75A‐AS1 and GAPLINC regulate polarization and innate immune responses. Inflamm Res. 2024;73(5):771‐792. [DOI] [PubMed] [Google Scholar]
- 204. Valverde A, Naqvi RA, Naqvi AR. Non‐coding RNA LINC01010 regulates macrophage polarization and innate immune functions by modulating NFκB signaling pathway. J Cell Physiol. 2024;239(5):e31225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cieplik F, Deng D, Crielaard W, et al. Antimicrobial photodynamic therapy – what we know and what we don't. Crit Rev Microbiol. 2018;44(5):571‐589. [DOI] [PubMed] [Google Scholar]
- 206. Elsadek MF. Clinical and bacterial outcomes of photodynamic therapy in the treatment of chronic necrotizing ulcerative periodontitis. Photodiagnosis Photodyn Ther. 2022;39:102977. [DOI] [PubMed] [Google Scholar]
- 207. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998;42(1):13‐28. [DOI] [PubMed] [Google Scholar]
- 208. Akram Z, Raffat MA, Saad Shafqat S, Mirza S, Ikram S. Clinical efficacy of photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis among cigarette smokers: a systematic review and meta‐analysis. Photodiagnosis Photodyn Ther. 2019;26:334‐341. [DOI] [PubMed] [Google Scholar]
- 209. Moreira AL, Novaes AB Jr, Grisi MF, et al. Antimicrobial photodynamic therapy as an adjunct to non‐surgical treatment of aggressive periodontitis: a split‐mouth randomized controlled trial. J Periodontol. 2015;86(3):376‐386. [DOI] [PubMed] [Google Scholar]
- 210. Lulic M, Leiggener Görög I, Salvi GE, Ramseier CA, Mattheos N, Lang NP. One‐year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof‐of‐principle randomized‐controlled clinical trial. J Clin Periodontol. 2009;36(8):661‐666. [DOI] [PubMed] [Google Scholar]
- 211. Ramanauskaite E, Moraschini V, Machiulskiene V, Sculean A. Clinical efficacy of single and multiple applications of antimicrobial photodynamic therapy in periodontal maintenance: a systematic review and network meta‐analysis. Photodiagnosis Photodyn Ther. 2021;36:102435. [DOI] [PubMed] [Google Scholar]
- 212. Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J Periodontol. 2009;80:1790‐1798. [DOI] [PubMed] [Google Scholar]
- 213. Séguier S, Souza SL, Sverzut AC, et al. Impact of photodynamic therapy on inflammatory cells during human chronic periodontitis. J Photochem Photobiol B. 2010;101:348‐354. [DOI] [PubMed] [Google Scholar]
- 214. Gholami L, Shahabi S, Jazaeri M, Hadilou M, Fekrazad R. Clinical applications of antimicrobial photodynamic therapy in dentistry. Front Microbiol. 2023;13:1020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Koduganti RR, Sandeep N, Guduguntla S, Chandana Gorthi VS. Probiotics and prebiotics in periodontal therapy. Indian J Dent Res. 2011;22(2):324‐330. [DOI] [PubMed] [Google Scholar]
- 216. Mack DR. Probiotics‐mixed messages. Can Fam Physician. 2005;51(11):1455‐1457, 1462–4. [PMC free article] [PubMed] [Google Scholar]
- 217. Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. The adjunctive use of host modulators in non‐surgical periodontal therapy. A systematic review of randomized, placebo‐controlled clinical studies. J Clin Periodontol. 2020;47(Suppl 22):199‐238. [DOI] [PubMed] [Google Scholar]
- 218. Kuru BE, Laleman I, Yalnızoğlu T, Kuru L, Teughels W. The influence of a Bifidobacterium animalis probiotic on gingival health: a randomized controlled clinical trial. J Periodontol. 2017;88(11):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 219. Iniesta M, Herrera D, Montero E, et al. Probiotic effects of orally administered lactobacillus reuteri‐containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J Clin Periodontol. 2012;39(8):736‐744. [DOI] [PubMed] [Google Scholar]
- 220. Ho SN, Acharya A, Sidharthan S, et al. A systematic review and meta‐analysis of clinical, immunological, and microbiological shift in periodontitis after nonsurgical periodontal therapy with adjunctive use of probiotics. J Evid Based Dent Pract. 2020;20(1):101397. [DOI] [PubMed] [Google Scholar]
- 221. Fu YW, Li XX, Xu HZ, Gong YQ, Yang Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: a randomized controlled trial. Clin Oral Investig. 2016;20(6):1263‐1269. [DOI] [PubMed] [Google Scholar]
- 222. Li YH, Ueng KC, Jeng JS, et al. Writing group of 2017 Taiwan lipid guidelines for high risk patients. 2017 Taiwan lipid guidelines for high‐risk patients. J Formos Med Assoc. 2017;116(4):217‐248. [DOI] [PubMed] [Google Scholar]
- 223. Liao JK. Effects of statins on 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibition beyond low‐density lipoprotein cholesterol. Am J Cardiol. 2005;96(5A):24F‐33F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Muniz FWMG, Taminski K, Cavagni J, Celeste RK, Weidlich P, Rösing CK. The effect of statins on periodontal treatment‐a systematic review with meta‐analyses and meta‐regression. Clin Oral Investig. 2018;22(2):671‐687. [DOI] [PubMed] [Google Scholar]
- 225. Wang AY, Lin GL, Keller JJ, Wang LH. Association between antihyperlipidemic agents and the risk of chronic periodontitis in patients with hyperlipidemia: a population‐based retrospective cohort study in Taiwan. J Periodontol. 2024;95(5):483‐493. [DOI] [PubMed] [Google Scholar]
- 226. Chapple IL. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J Am Dent Assoc. 2009;140(2):178‐184. [DOI] [PubMed] [Google Scholar]
- 227. Stańdo‐Retecka M, Piatek P, Namiecinska M, Bonikowski R, Lewkowicz P, Lewkowicz N. Clinical and microbiological outcomes of subgingival instrumentation supplemented with high‐dose omega‐3 polyunsaturated fatty acids in periodontal treatment – a randomized clinical trial. BMC Oral Health. 2023;23(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kruse AB, Kowalski CD, Leuthold S, Vach K, Ratka‐Krüger P, Woelber JP. What is the impact of the adjunctive use of omega‐3 fatty acids in the treatment of periodontitis? A systematic review and meta‐analysis. Lipids Health Dis. 2020;19(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Van Ravensteijn MM, Timmerman MF, Brouwer EAG, Slot DE. The effect of omega‐3 fatty acids on active periodontal therapy: a systematic review and meta‐analysis. J Clin Periodontol. 2022;49(10):1024‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Stańdo M, Piatek P, Namiecinska M, Lewkowicz P, Lewkowicz N. Omega‐3 polyunsaturated fatty acids EPA and DHA as an adjunct to non‐surgical treatment of periodontitis: a randomized clinical trial. Nutrients. 2020;12(9):2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Tonetti MS, Chapple IL. Working group 3 of seventh European workshop on periodontology. Biological approaches to the development of novel periodontal therapies–consensus of the seventh European workshop on periodontology. J Clin Periodontol. 2011;38(Suppl 11):114‐118. [DOI] [PubMed] [Google Scholar]
- 232. Tomofuji T, Ekuni D, Sanbe T, et al. Effects of vitamin C intake on gingival oxidative stress in rat periodontitis. Free Radic Biol Med. 2009;46(2):163‐168. [DOI] [PubMed] [Google Scholar]
- 233. Neiva RF, Al‐Shammari K, Nociti FH Jr, Soehren S, Wang HL. Effects of vitamin‐B complex supplementation on periodontal wound healing. J Periodontol. 2005;76(7):1084‐1091. [DOI] [PubMed] [Google Scholar]
- 234. Toubi E, Shoenfeld Y. The role of vitamin D in regulating immune responses. Isr Med Assoc J. 2010;12(3):174‐175. [PubMed] [Google Scholar]
- 235. Akbari S, Rasouli‐Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int. 2018;2018:4629383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Akedo Y, Hosoi T, Inoue S, et al. Vitamin K2 modulates proliferation and function of osteoblastic cells in vitro. Biochem Biophys Res Commun. 1992;187(2):814‐820. [DOI] [PubMed] [Google Scholar]
- 237. Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol. 2003;176(3):339‐348. [DOI] [PubMed] [Google Scholar]
- 238. Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by BioMed Research international suppressing NF‐kappaB activation. Int J Mol Med. 2011;27(1):3‐14. [DOI] [PubMed] [Google Scholar]
- 239. Aral K, Alkan BA, Saraymen R, Yay A, Şen A, Önder GÖ. Therapeutic effects of systemic vitamin k2 and vitamin d3 on gingival inflammation and alveolar bone in rats with experimentally induced periodontitis. J Periodontol. 2015;86(5):666‐673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data were used in this manuscript.


