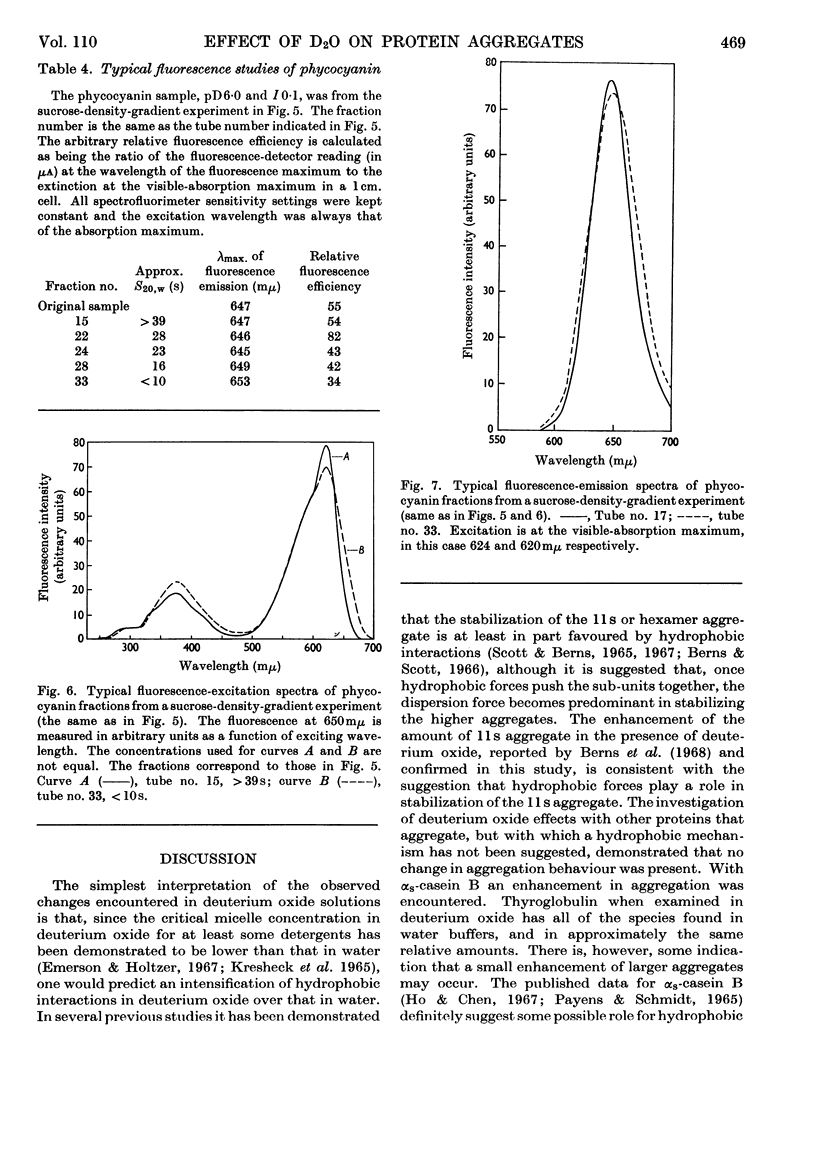
Abstract
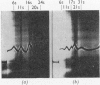
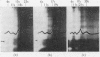
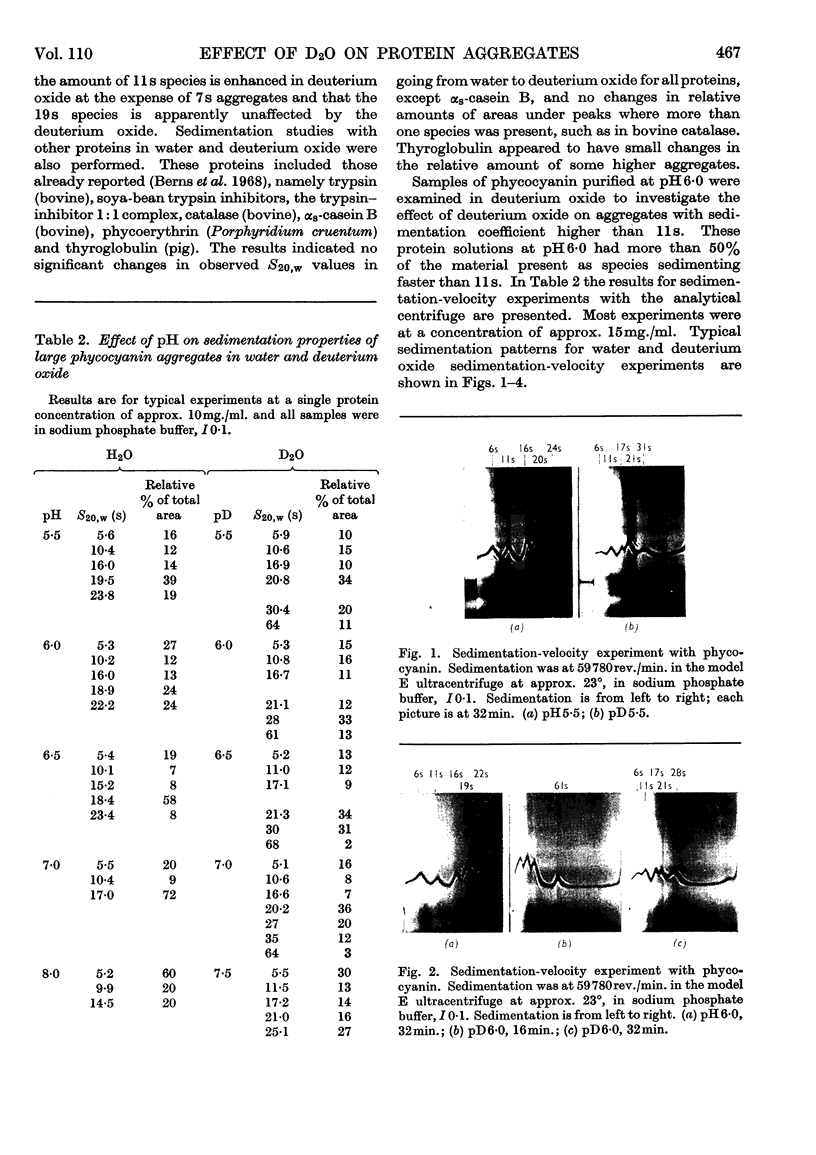
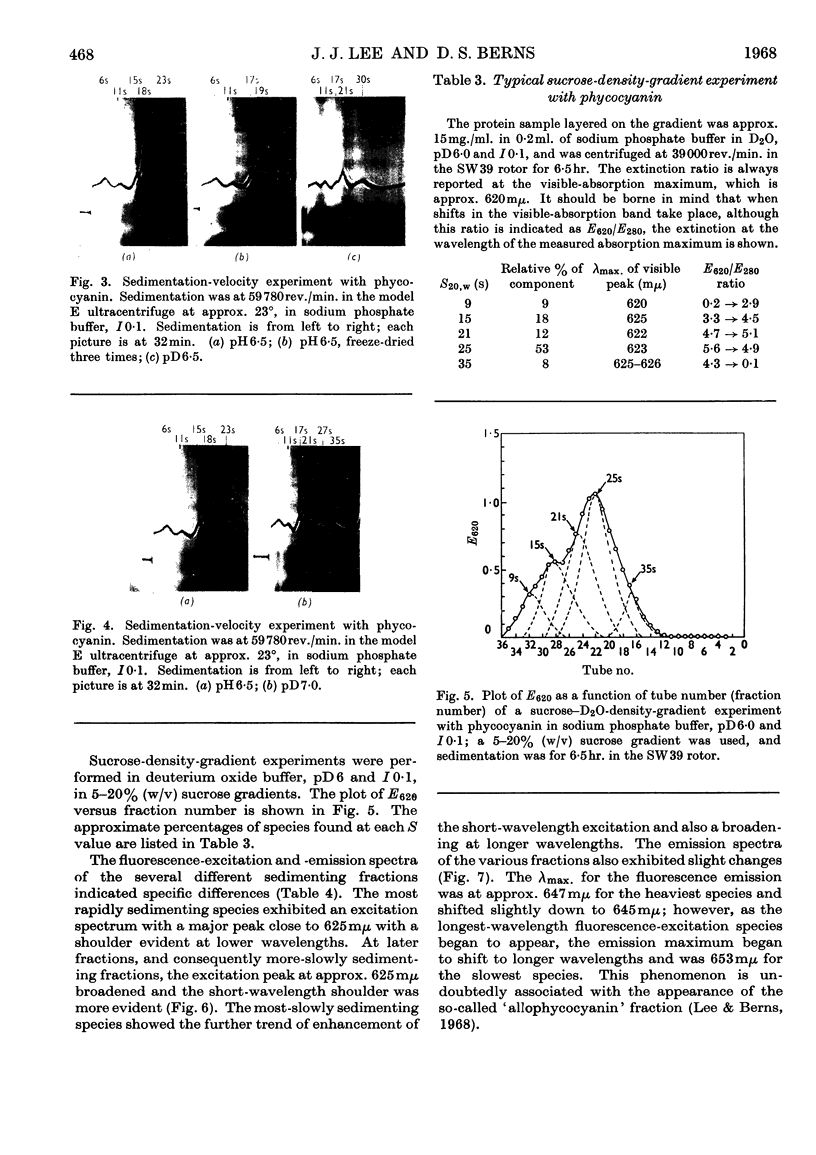
The amount of 11s aggregate in phycocyanin, normally stimulated by hydrophobic forces, is dramatically increased by the presence of deuterium oxide. Proteins in which hydrophobic forces are not proposed as a mechanism for aggregation are unaffected by deuterium oxide. These observations are consistent with the lower critical micelle concentration reported for ionic detergents in deuterium oxide. Phycocyanin samples containing a majority of material sedimenting faster than 11s were also investigated in the presence of deuterium oxide with the following findings: the most rapidly sedimenting species in water buffer is 24s; in deuterium oxide more than 10% of the protein sediments at 67s and substantial amounts of other species with sedimentation coefficients larger than 24s are present. These large quantities of species sedimenting faster than 24s are found in deuterium oxide buffers from pD5·5 to 7·0. Sucrose-density-gradient studies in deuterium oxide at pD6·0 confirm the presence of large amounts of more rapidly sedimenting species. Spectrophotometric studies on fractions from the sucrose-density-gradient experiments indicate with the presence of higher aggregates a red shift of the visible-absorption maximum and an enhancement of the E620/E280 ratio. Fluorescence-emission studies show a greater relative fluorescence efficiency for these higher aggregates and are consistent with the suggested enhancement of higher aggregates in deuterium oxide. The existence of phycocyanin aggregates of such a large size is suggested to be of importance in vivo, with phycocyanin playing a role as a structural protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns D. S., Scott E. Protein aggregation in a thermophilic protein. Phycocyanin from Synechococcus lividus. Biochemistry. 1966 May;5(5):1528–1533. doi: 10.1021/bi00869a012. [DOI] [PubMed] [Google Scholar]
- EDELHOCH H. The properties of thyroglobulin. I. The effects of alkali. J Biol Chem. 1960 May;235:1326–1334. [PubMed] [Google Scholar]
- Edelhoch H., De Crombrugghe B. The properties of thyroglobulin. 13. The structure of reduced alkylated thyroglobulin. J Biol Chem. 1966 Oct 10;241(19):4357–4365. [PubMed] [Google Scholar]
- Edelstein S. J., Schachman H. K. The simultaneous determination of partial specific volumes and molecular weights with microgram quantities. J Biol Chem. 1967 Jan 25;242(2):306–311. [PubMed] [Google Scholar]
- Emerson M. F., Holtzer A. The hydrophobic bond in micellar systems. Effects of various additives on the stability of micelles of sodium dodecyl sulfate and of n-dodecyltrimethylammonium bromide. J Phys Chem. 1967 Sep;71(10):3320–3330. doi: 10.1021/j100869a031. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- Ho C., Chen A. H. The polymerization of bovine alpha-s-casein B. J Biol Chem. 1967 Feb 10;242(3):551–553. [PubMed] [Google Scholar]
- Kresheck G. C., Schneider H., Scheraga H. A. The effect of D2-O on the thermal stability of proteins. Thermodynamic parameters for the transfer of model compounds from H2-O to D2-O. J Phys Chem. 1965 Sep;69(9):3132–3144. doi: 10.1021/j100893a054. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Berns D. S. Protein aggregation. Studies of larger aggregates of C-phycocyanin. Biochem J. 1968 Dec;110(3):457–464. doi: 10.1042/bj1100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Morphology of a virus of blue-green algae and properties of its deoxyribonucleic acid. J Virol. 1967 Apr;1(2):344–361. doi: 10.1128/jvi.1.2.344-361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Paglini S., Lauffer M. A. Polymerization-depolymerization of tobacco mosaic virus protein. XI. Osmotic pressure studies of solutions in water and in deuterium. Biochemistry. 1968 May;7(5):1827–1835. doi: 10.1021/bi00845a030. [DOI] [PubMed] [Google Scholar]
- Payens T. A., Schmidt D. G. The thermodynamic parameters of the association of alpha-s-casein C. Biochim Biophys Acta. 1965 Sep 27;109(1):214–222. doi: 10.1016/0926-6585(65)90105-6. [DOI] [PubMed] [Google Scholar]
- Scott E., Berns D. S. Completely deuterated proteins. 3. Deuteration effects on protein-protein interaction in phycocyanin. Biochemistry. 1967 May;6(5):1327–1334. doi: 10.1021/bi00857a015. [DOI] [PubMed] [Google Scholar]
- Scott E., Berns D. S. Protein-protein interaction. The phycocyanin system. Biochemistry. 1965 Dec;4(12):2597–2606. doi: 10.1021/bi00888a008. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]