Abstract
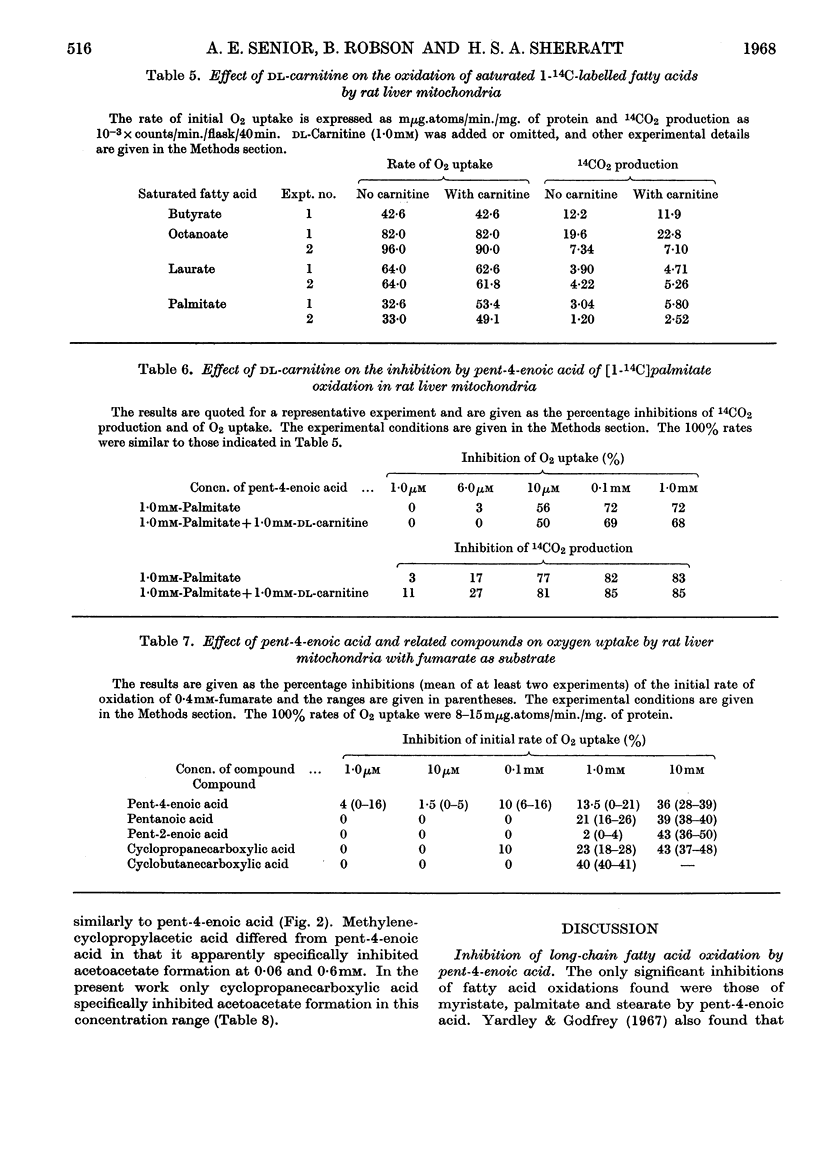
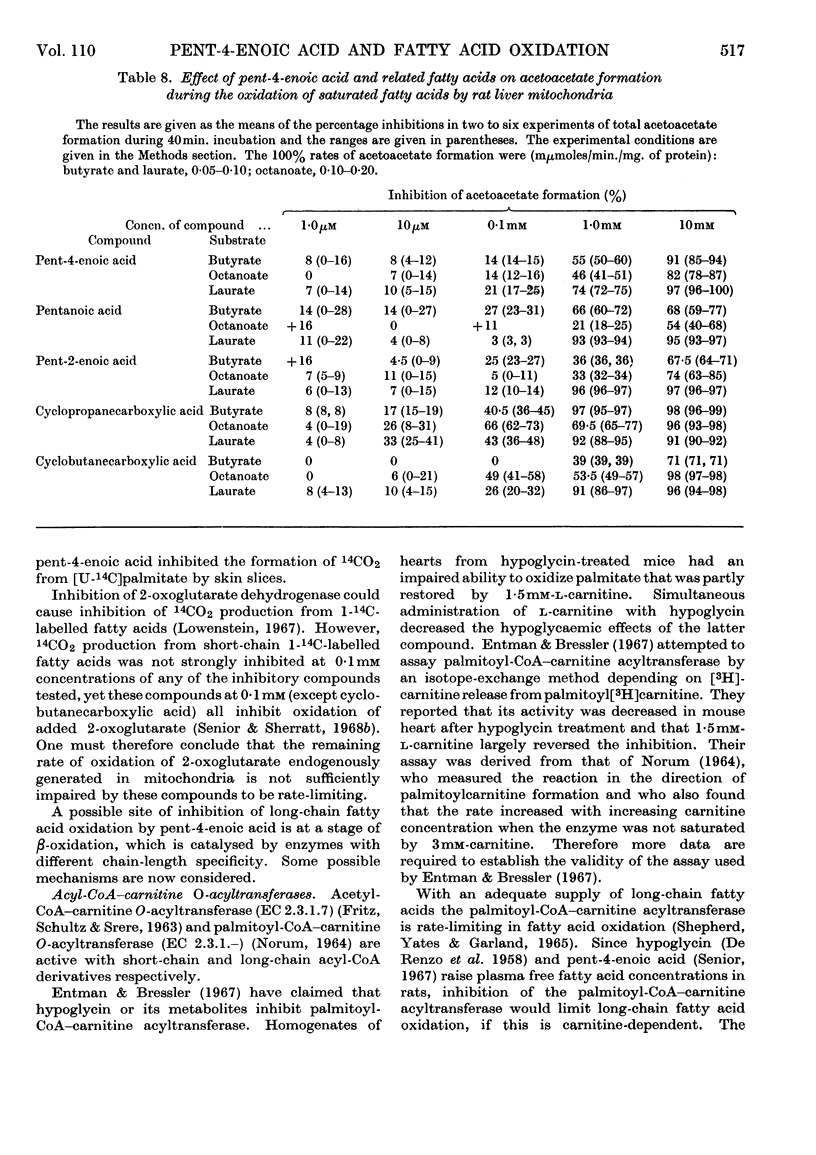
1. The effects of the hypoglycaemic compound, pent-4-enoic acid, and of four structurally related non-hypoglycaemic compounds (pentanoic acid, pent-2-enoic acid, cyclopropanecarboxylic acid and cyclobutanecarboxylic acid), on the oxidation of saturated fatty acids by rat liver mitochondria were determined. 2. The formation of 14CO2 from [1-14C]palmitate was strongly inhibited by 0·01mm-pent-4-enoic acid. 3. The inhibition of oxygen uptake was less than that of 14CO2 formation, presumably because fumarate was used as a sparker. 4. The oxidation of [1-14C]-butyrate, -octanoate or -laurate was not strongly inhibited by 0·01mm-pent-4-enoic acid. 5. The other four non-hypoglycaemic compounds did not inhibit the oxidation of any saturated fatty acid when tested at 0·01mm concentration, though they all inhibited strongly at 10mm. 6. The oxidation of [1-14C]-myristate and -stearate, but not of [1-14C]decanoate, was strongly inhibited by 0·01mm-pent-4-enoic acid. 7. The oxidation of [1-14C]palmitate was about 50% carnitine-dependent under the experimental conditions used. 8. The percentage inhibition of [1-14C]palmitate oxidation by pent-4-enoic acid was the same whether carnitine was present or not. 9. Acetoacetate formation from saturated fatty acids was inhibited by 0·1mm-cyclopropanecarboxylic acid to a greater extent than their oxidation. 10. The other compounds tested inhibited acetoacetate formation from saturated fatty acids proportionately to the inhibition of oxidation. 11. Possible mechanisms for the inhibition of long-chain fatty acid oxidation by pent-4-enoic acid are discussed. 12. There was a correlation between the ability to inhibit long-chain fatty acid oxidation and hypoglycaemic activity in this series of compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., SCHOLEFIELD P. G. Studies of fatty acid oxidation. 7. The effects of fatty acids on the phosphate metabolism of slice and mitochondrial preparations of rat liver. Biochem J. 1961 Oct;81:37–45. doi: 10.1042/bj0810037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BJOERNTORP P., ELLS H. A., BRADFORD R. H. ALBUMIN ANTAGONISM OF FATTY ACID EFFECTS ON OXIDATION AND PHOSPHORYLATION REACTIONS IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Jan;239:339–344. [PubMed] [Google Scholar]
- Corredor C., Brendel K., Bressler R. Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2299–2306. doi: 10.1073/pnas.58.6.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman M., Bressler R. The mechanism of action of hypoglycin on long-chain fatty acid oxidation. Mol Pharmacol. 1967 Jul;3(4):333–340. [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- Galzigna L., Rossi C. R., Sartorelli L., Gibson D. M. A guanosine triphosphate-dependent acyl coenzyme A synthetase from rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2111–2115. [PubMed] [Google Scholar]
- NORUM K. R. PALMITYL-COA:CARNITINE PALMITYLTRANSFERASE. PURIFICATION FROM CALF-LIVER MITOCHONDRIA AND SOME PROPERTIES OF THE ENZYME. Biochim Biophys Acta. 1964 Jul 8;89:95–108. [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Carbohydrate metabolism. Biochem J. 1968 Dec;110(3):521–527. doi: 10.1042/bj1100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Oxidative phosphorylation and mitochondrial oxidation of pyruvate, 3-hydroxybutyrate and tricarboxylic acid-cycle intermediates. Biochem J. 1968 Dec;110(3):499–509. doi: 10.1042/bj1100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. The effect of pent-4-enoic acid and some simple related compounds on the oxidation of fatty acids by rat-liver mitochondria. Biochem J. 1967 Sep;104(3):56P–56P. [PMC free article] [PubMed] [Google Scholar]
- Von Holt C. Methylenecyclopropaneacetic acid, a metabolite of hypoglycin. Biochim Biophys Acta. 1966 Aug 3;125(1):1–10. doi: 10.1016/0005-2760(66)90138-x. [DOI] [PubMed] [Google Scholar]
- Von Holt C., Von Holt M., Böhm H. Metabolic effects of hypoglycin and methylenecyclopropaneacetic acid. Biochim Biophys Acta. 1966 Aug 3;125(1):11–21. doi: 10.1016/0005-2760(66)90139-1. [DOI] [PubMed] [Google Scholar]
- Yardley H. J., Godfrey G. Some in vitro effects of hypoglycin on skin. Arch Dermatol. 1967 Jul;96(1):89–93. [PubMed] [Google Scholar]
- Yates D. W., Shepherd D., Garland P. B. Organization of fatty-acid activation in rat liver mitochondria. Nature. 1966 Mar 19;209(5029):1213–1215. doi: 10.1038/2091213a0. [DOI] [PubMed] [Google Scholar]


