Abstract
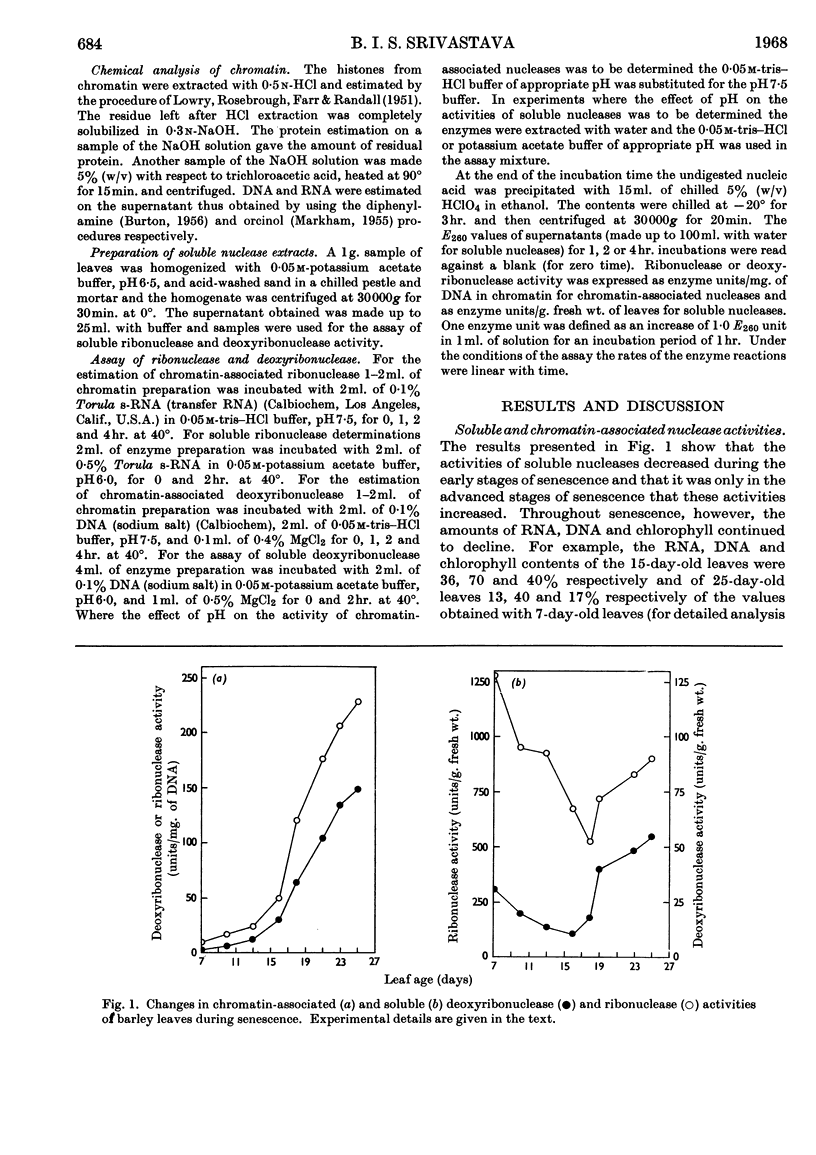
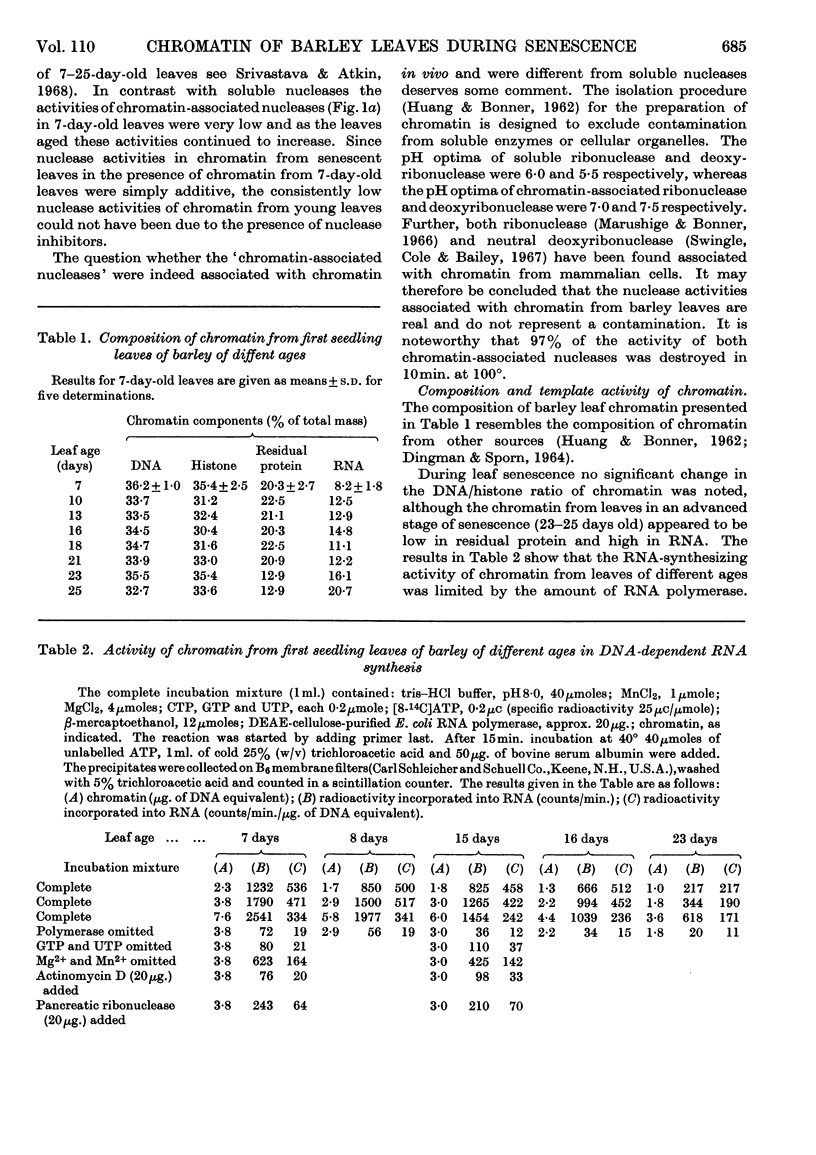
1. The activity of soluble ribonuclease and deoxyribonuclease first declined during senescence, but later increased during advanced stages of senescence. 2. Young leaves had very low ribonuclease or deoxyribonuclease activity associated with the chromatin, but the activity of these enzymes increased progressively during senescence until the leaves died. 3. No significant changes in the composition of chromatin from first seedling leaves of barley plants during aging (from 7 to 25 days) were noted. 4. The amount of RNA synthesized by chromatin in vitro declined as the leaf aged. However, if the loss of RNA due to chromatin-associated ribonuclease was taken into account, the RNA-synthesizing activity of chromatin from senescing (15–16-day-old) leaves appeared to be somewhat higher than that of chromatin from young (7–8-day-old) leaves. In leaves at the terminal stages of senescence (23 days old) the estimates of RNA synthesis by chromatin could not be made owing to complications created by high nuclease activities. 5. It is suggested that senescence may be triggered by a decline in some hormonal factor in leaves, and that the resulting production of chromatin-associated deoxyribonuclease and ribonuclease in increasing proportions may progressively cause increased degradation of DNA and newly synthesized RNA, so that ultimately the cellular functions are impaired and the cells die.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkas J. D., Chargaff E. Template functions in the enzymic formation of polyribonucleotides. IV. Denatured DNA as template. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1645–1651. doi: 10.1073/pnas.58.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Atkin R. K. Effect of second-leaf removal or kinetin treatment on the nucleic acid metabolism of senescing first seedling leaf of barley. Biochem J. 1968 Apr;107(3):361–366. doi: 10.1042/bj1070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B. I. Cytokinins in plants. Int Rev Cytol. 1967;22:349–387. doi: 10.1016/s0074-7696(08)61839-2. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Increase in chromatin associated nuclease ctivity of excised barley leaves during senescence and its suppression by kinetin. Biochem Biophys Res Commun. 1968 Aug 13;32(3):533–538. doi: 10.1016/0006-291x(68)90695-5. [DOI] [PubMed] [Google Scholar]
- Stout E. R., Mans R. J. Template requirement of maize RNA polymerase. Plant Physiol. 1968 Mar;43(3):405–410. doi: 10.1104/pp.43.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingle K. F., Cole L. J., Bailey J. S. Association of neutral deoxyribonuclease with chromatin isolated from mammalian cells. Biochim Biophys Acta. 1967 Dec 19;149(2):467–475. doi: 10.1016/0005-2787(67)90174-8. [DOI] [PubMed] [Google Scholar]


