Abstract
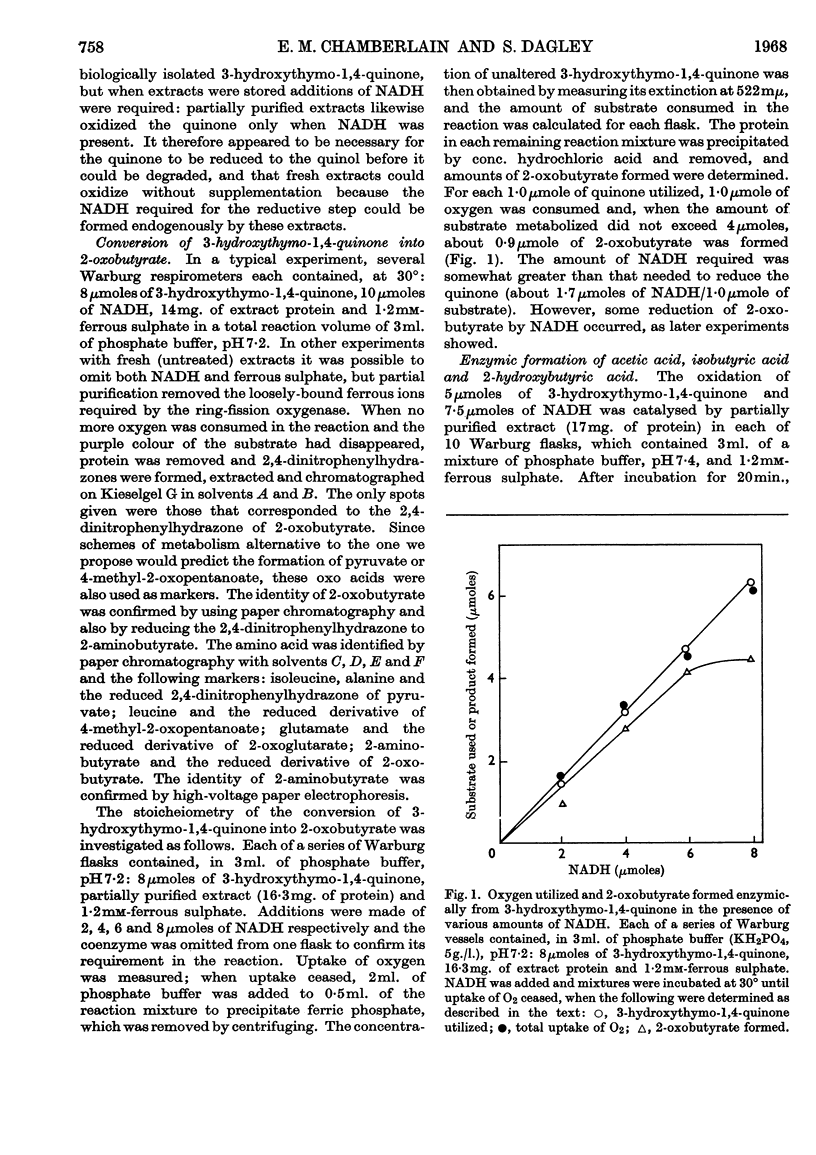
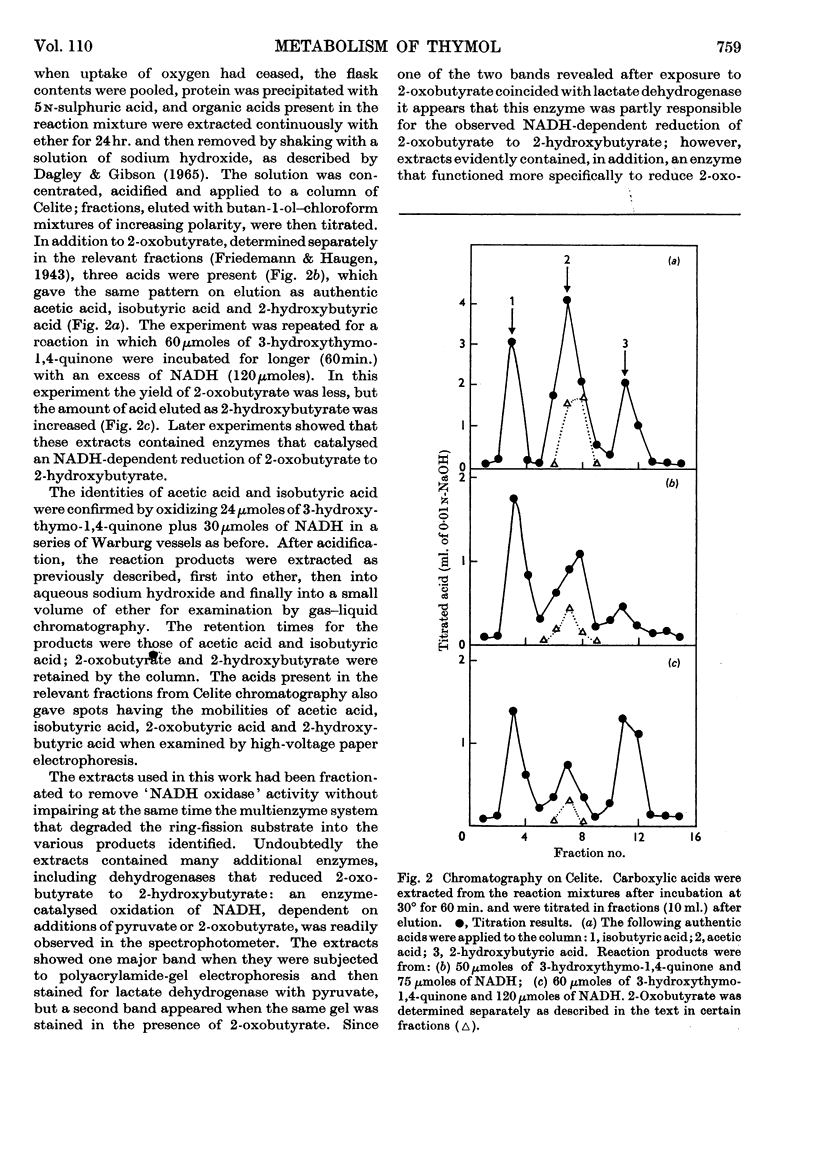
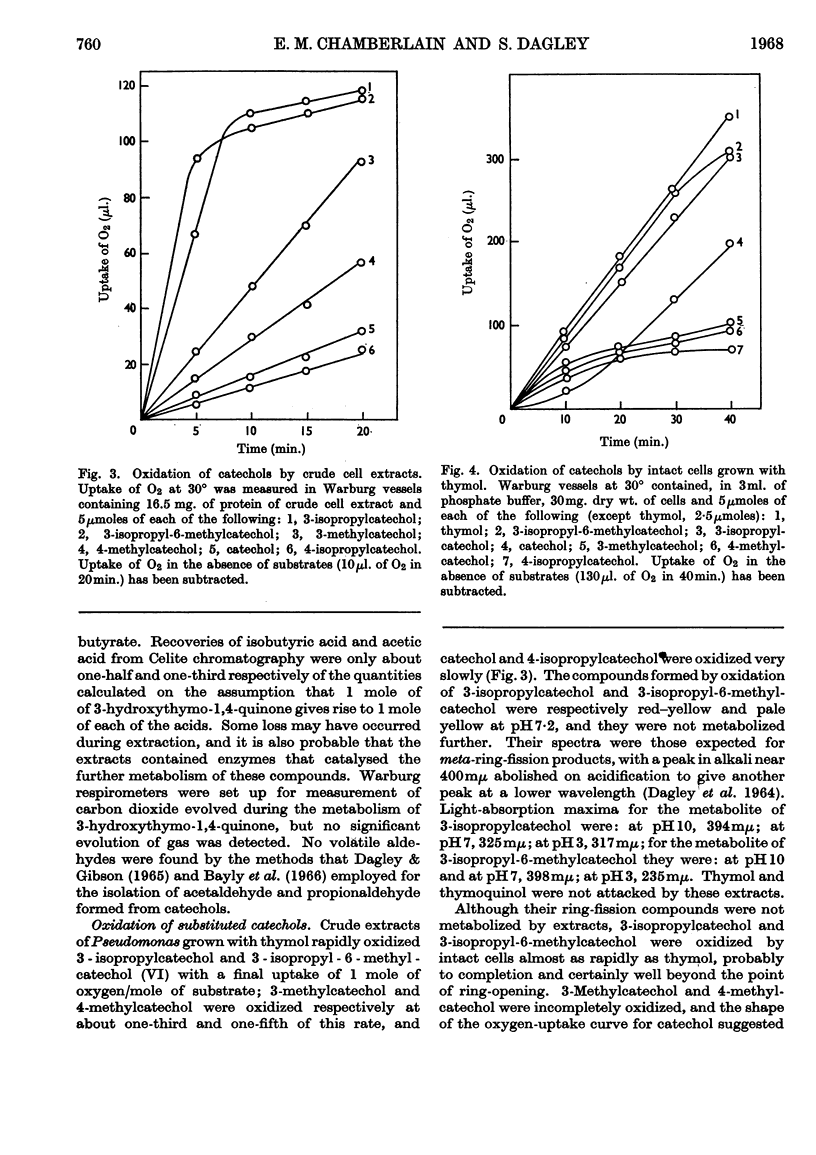
1. Pseudomonas putida when grown with thymol contained a meta-fission dioxygenase, which required ferrous ions and readily cleaved the benzene nucleus of catechols between adjacent carbon atoms bearing hydroxyl and isopropyl groups. 2. 3-Hydroxythymo-1,4-quinone was excreted towards the end of exponential growth and later was slowly metabolized. This compound was oxidized by partially purified extracts only when NADH was supplied; the substrate for the dioxygenase appeared to be 3-hydroxythymo-1,4-quinol, which was readily and non-enzymically oxidized to the quinone. 3. 2-Oxobutyrate (0·9 mole) was formed from 1 mole of 3-hydroxythymo-1,4-quinone with the consumption of 1 mole of oxygen; acetate, isobutyrate and 2-hydroxybutyrate (which arose from the enzymic reduction of 2-oxobutyrate) were also formed. 4. These products, which were produced only when the catechol substrate contained a third hydroxyl group, appeared to result from the enzymic hydrolysis of the ring-fission product.
Full text
PDF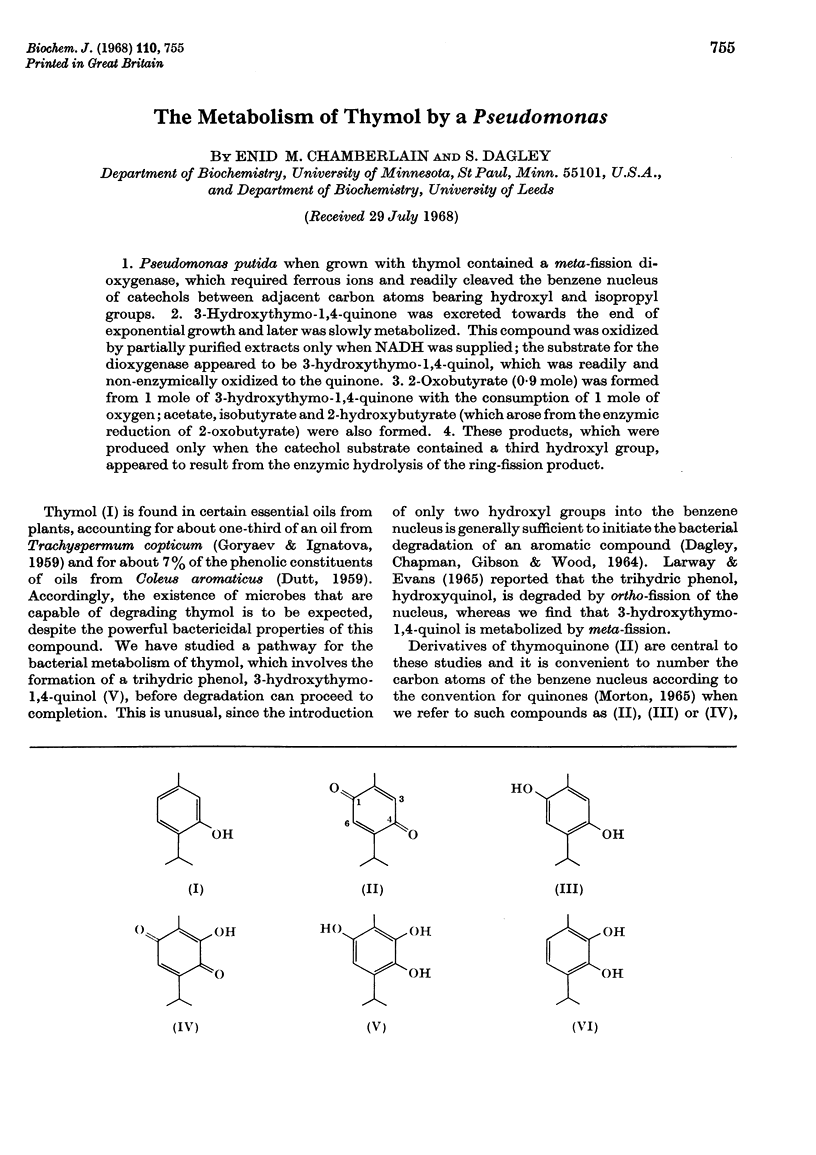




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF GALACTARATE, D-GLUCARATE AND VARIOUS PENTOSES BY SPECIES OF PSEUDOMONAS. Biochem J. 1965 Apr;95:48–58. doi: 10.1042/bj0950048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSALL H., GREENBERG D. M. Studies on the enzymic decomposition of urocanic acid. V. The formation of 4-oxoglutaramic acid, a nonenzymic oxidation product of 4(5)-imidazolone-5(4)-propionic acid. J Biol Chem. 1963 Apr;238:1423–1431. [PubMed] [Google Scholar]
- HAYAISHI O., TANIUCHI H., TASHIRO M., KUNO S. Studies on the metabolism of kynurenic acid. I. The formation of L-glutamic acid, D- and L-alanine, and acetic acid from kynurenic acid by Pseudomonas extracts. J Biol Chem. 1961 Sep;236:2492–2497. [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- MORRISON R. I. The isolation of L-pipecolinic acid from Trifolium repens. Biochem J. 1953 Feb;53(3):474–478. doi: 10.1042/bj0530474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]


