Abstract
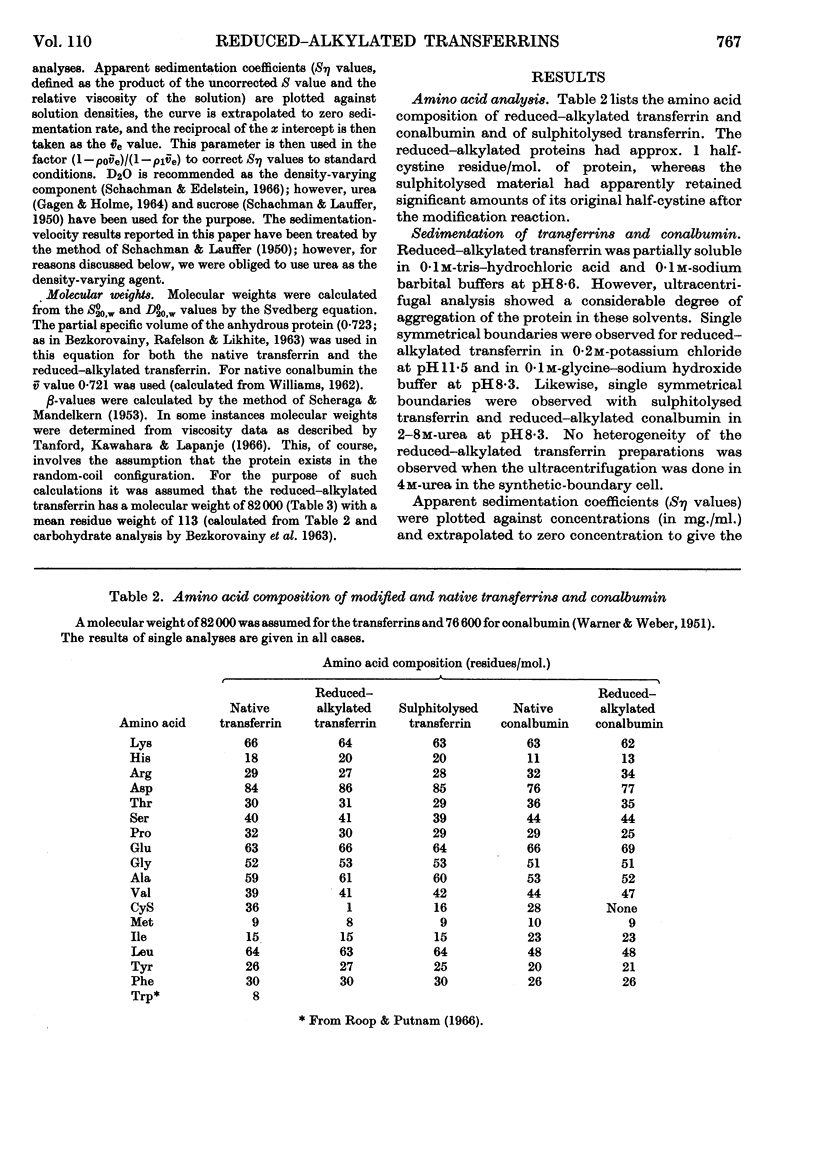
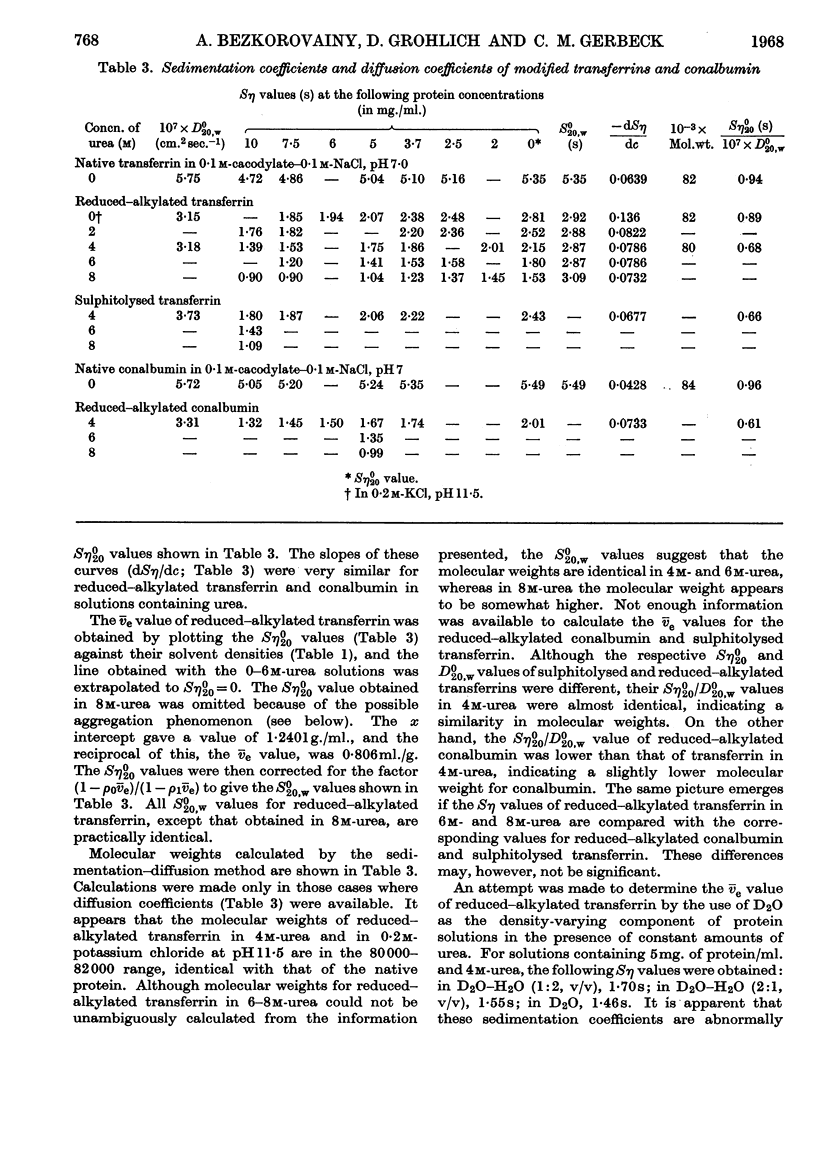
1. Apparently all disulphide bridges of transferrin and conalbumin were broken by reduction–alkylation, whereas sulphitolysis resulted in incomplete cleavage of disulphide bonds. 2. The molecular weights of reduced–alkylated and sulphitolysed transferrin and reduced–alkylated conalbumin were identical with those of native proteins in a number of solvents, indicating that these proteins exist as single polypeptide chains. 3. Viscosity studies indicated that reduced–alkylated transferrin possesses a partially ordered structure in 0–4m-urea, assumes a random-coil configuration in 6m-urea with a molecular weight of 84000 and is partially aggregated in 8m-urea.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- BEZKOROVAINY A., RAFELSON M. E., Jr, LIKHITE V. ISOLATION AND PARTIAL CHARACTERIZATION OF TRANSFERRIN FROM NORMAL HUMAN PLASMA. Arch Biochem Biophys. 1963 Dec;103:371–378. doi: 10.1016/0003-9861(63)90427-2. [DOI] [PubMed] [Google Scholar]
- Bezkorovainy A. Comparative study of metal-free, iron-saturated and sialic acid-free transferrins. Biochim Biophys Acta. 1966 Oct 31;127(2):535–537. doi: 10.1016/0304-4165(66)90410-7. [DOI] [PubMed] [Google Scholar]
- Bezkorovainy A., Grohlich D. The behavior of nativ and reduced-alkylated human transferrin in urea and guanidine-HCl solutions. Biochim Biophys Acta. 1967 Dec 12;147(3):497–510. doi: 10.1016/0005-2795(67)90009-8. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Gagen W. L. The significance of the "partial specific volume" obtained from sedimentation data. Biochemistry. 1966 Aug;5(8):2553–2557. doi: 10.1021/bi00872a010. [DOI] [PubMed] [Google Scholar]
- Hill J., Cox D. J. Studies of the sedimentation velocity of ovalbumin in concentrated salt solutions. J Phys Chem. 1965 Sep;69(9):3032–3040. doi: 10.1021/j100893a036. [DOI] [PubMed] [Google Scholar]
- JAMIESON G. A. STUDIES ON GLYCOPROTEINS. II. ISOLATION OF THE CARBOHYDRATE CHAINS OF HUMAN TRANSFERRIN. J Biol Chem. 1965 Jul;240:2914–2920. [PubMed] [Google Scholar]
- Jeppsson J. O. Subunits of human transferrin. Acta Chem Scand. 1967;21(7):1686–1694. doi: 10.3891/acta.chem.scand.21-1686. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Tanford C. Viscosity and density of aqueous solutions of urea and guanidine hydrochloride. J Biol Chem. 1966 Jul 10;241(13):3228–3232. [PubMed] [Google Scholar]
- Leibman A. J., Aisen P. Preparation of single crystals of transferrin. Arch Biochem Biophys. 1967 Sep;121(3):717–719. doi: 10.1016/0003-9861(67)90058-6. [DOI] [PubMed] [Google Scholar]
- Line W. F., Grohlich D., Bezkorovainy A. The effect of chemical modification on the iron binding properties of human transferrin. Biochemistry. 1967 Nov;6(11):3393–3402. doi: 10.1021/bi00863a009. [DOI] [PubMed] [Google Scholar]
- Noelken M. E., Timasheff S. N. Preferential solvation of bovine serum albumin in aqueous guanidine hydrochloride. J Biol Chem. 1967 Nov 10;242(21):5080–5085. [PubMed] [Google Scholar]
- Roberts R., Makey D. G., Seal U. S. Human transferrin. Molecular weight and sedimentation properties. J Biol Chem. 1966 Nov 10;241(21):4907–4913. [PubMed] [Google Scholar]
- Roop W. E., Putnam F. W. Purification and properties of human transferrin C and a slow moving genetic variant. J Biol Chem. 1967 May 25;242(10):2507–2513. [PubMed] [Google Scholar]
- SKEGGS L. T., Jr An automatic method for colorimetric analysis. Am J Clin Pathol. 1957 Sep;28(3):311–322. doi: 10.1093/ajcp/28.3_ts.311. [DOI] [PubMed] [Google Scholar]
- Schachman H. K., Edelstein S. J. Ultracentrifuge studies with absorption optics. IV. Molecular weight determinations at the microgram level. Biochemistry. 1966 Aug;5(8):2681–2705. doi: 10.1021/bi00872a029. [DOI] [PubMed] [Google Scholar]
- Tanford C., Kawahara K., Lapanje S. Proteins in 6-M guanidine hydrochloride. Demonstration of random coil behavior. J Biol Chem. 1966 Apr 25;241(8):1921–1923. [PubMed] [Google Scholar]
- Ullmann A., Goldberg M. E., Perrin D., Monod J. On the determination of molecular weight of proteins and protein subunits in the presence of 6 M guanidine hydrochloride. Biochemistry. 1968 Jan;7(1):261–265. doi: 10.1021/bi00841a031. [DOI] [PubMed] [Google Scholar]
- WARNER R. C., WEBER I. The preparation of crystalline conalbumin. J Biol Chem. 1951 Jul;191(1):173–180. [PubMed] [Google Scholar]
- WILLIAMS J. Serum proteins and the livetins of hen's-egg yolk. Biochem J. 1962 May;83:346–355. doi: 10.1042/bj0830346. [DOI] [PMC free article] [PubMed] [Google Scholar]


