Abstract
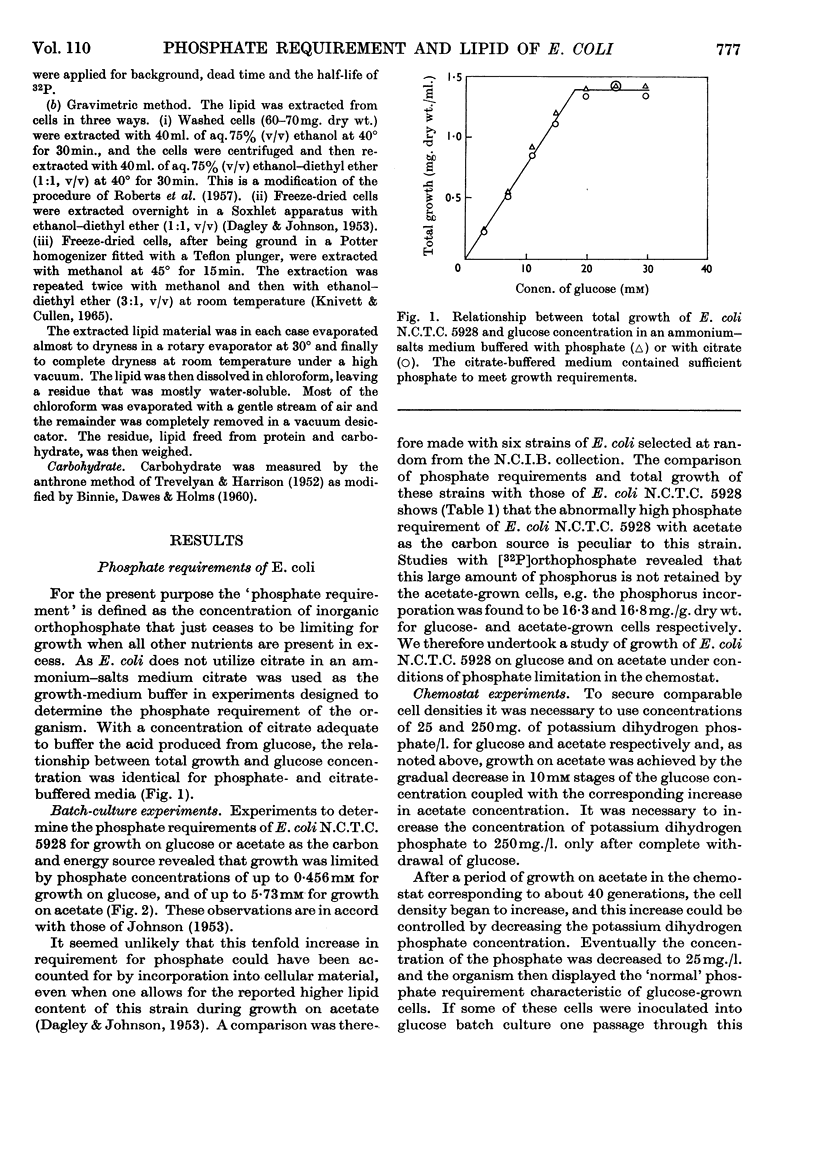
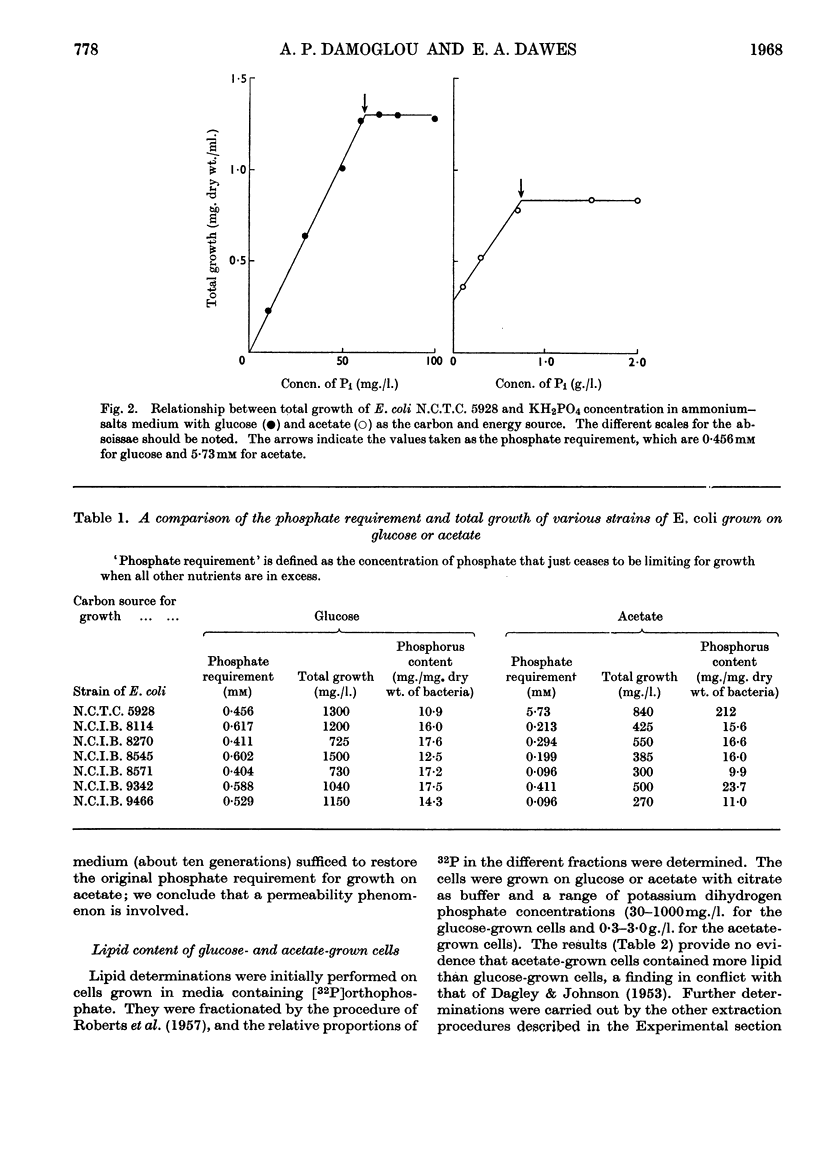
1. The phosphate requirement, i.e. the concentration of inorganic orthophosphate that just ceases to be limiting for growth, of Escherichia coli N.C.T.C. 5928 was determined for growth in ammonium–salts media containing glucose or acetate as the carbon and energy source, and compared with that of six other strains of E. coli. 2. The phosphate requirement for E. coli N.C.T.C. 5928 growing on acetate was about ten times that for growth on glucose, but this difference was not observed with any of the other strains. 3. After about 40 generations' growth on acetate with phosphate limitation in a chemostat, the phosphate requirement of the cells gradually decreased until it was equivalent to that of the glucose-grown organism; a single passage through glucose batch culture sufficed to restore the original high phosphate requirement, indicating a permeability phenomenon. 4. The lipid content of E. coli N.C.T.C. 5928 grown on glucose or acetate was measured isotopically by fractionation of cells grown on inorganic [32P]orthophosphate and gravimetrically after extraction from the cells by three different methods; change of carbon source from glucose to acetate did not affect the lipid content, which remained constant at 8–9% of the bacterial dry weight.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BINNIE B., DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. IV. Patterns of oxidative assimilation. Biochim Biophys Acta. 1960 May 20;40:237–251. doi: 10.1016/0006-3002(60)91348-2. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., DAWES E. A. Factors influencing the polysaccharide content of Escherichia coli. Biochem J. 1949;45(3):331–337. doi: 10.1042/bj0450331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., JOHNSON A. R. The relation between lipid and polysaccharide contents of Bact. coli. Biochim Biophys Acta. 1953 May;11(1):158–159. doi: 10.1016/0006-3002(53)90020-1. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoglou A. P., Dawes E. A. Effect of phosphate limitation on the senescence of glucose-and acetate-grown Escherichia coli. Biochem J. 1967 Sep;104(3):48P–49P. [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Some factors affecting cyclopropane acid formation in Escherichia coli. Biochem J. 1965 Sep;96(3):771–776. doi: 10.1042/bj0960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]


