Abstract
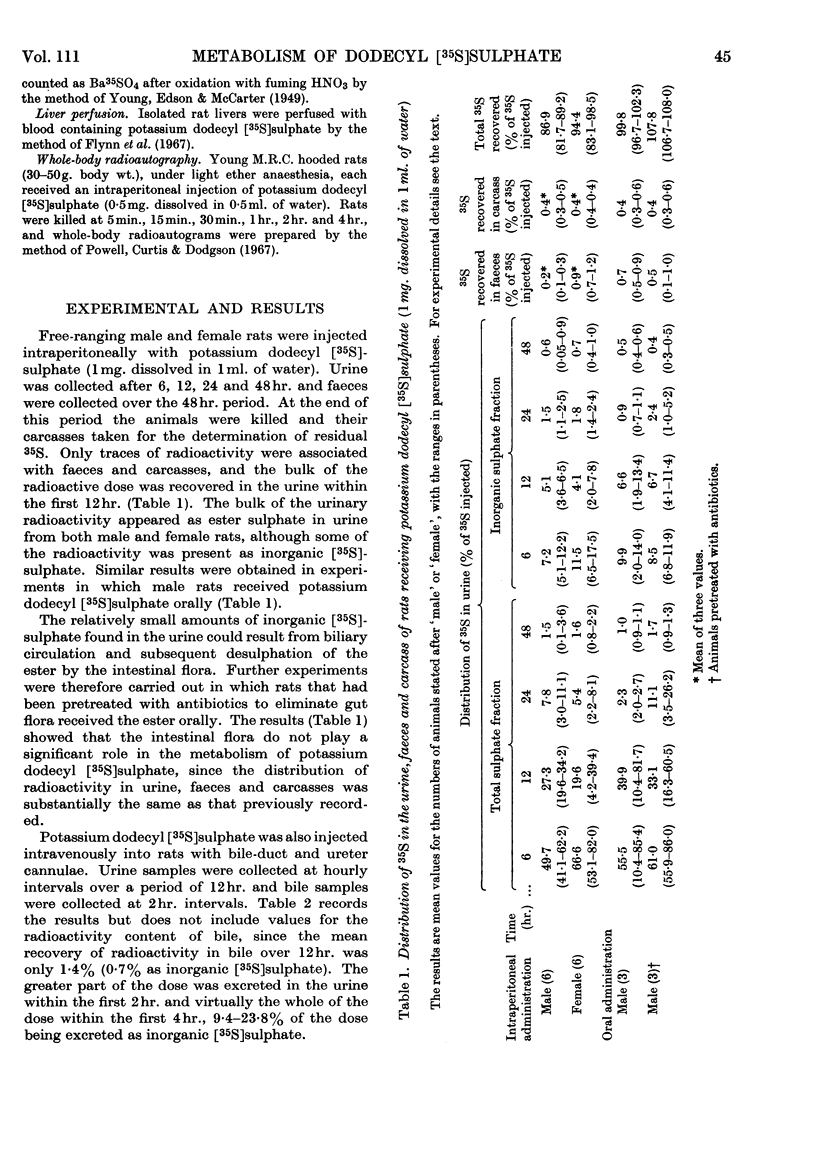
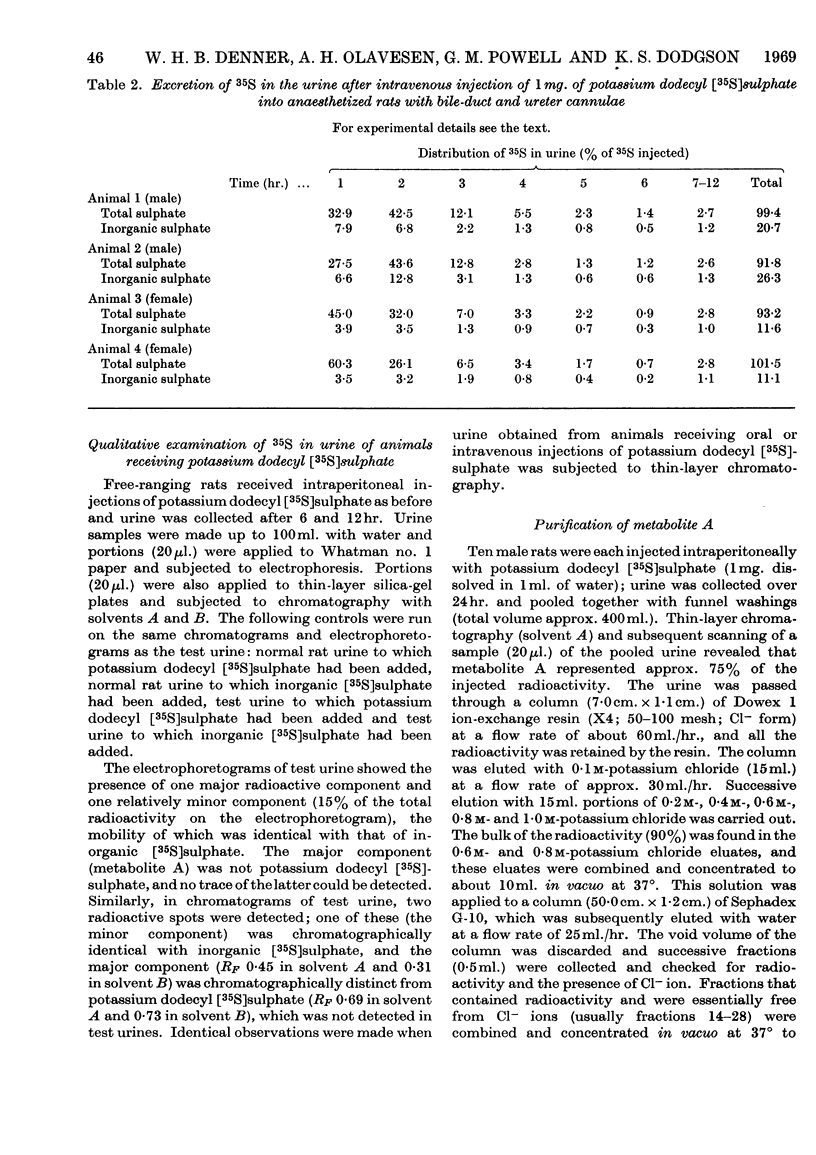
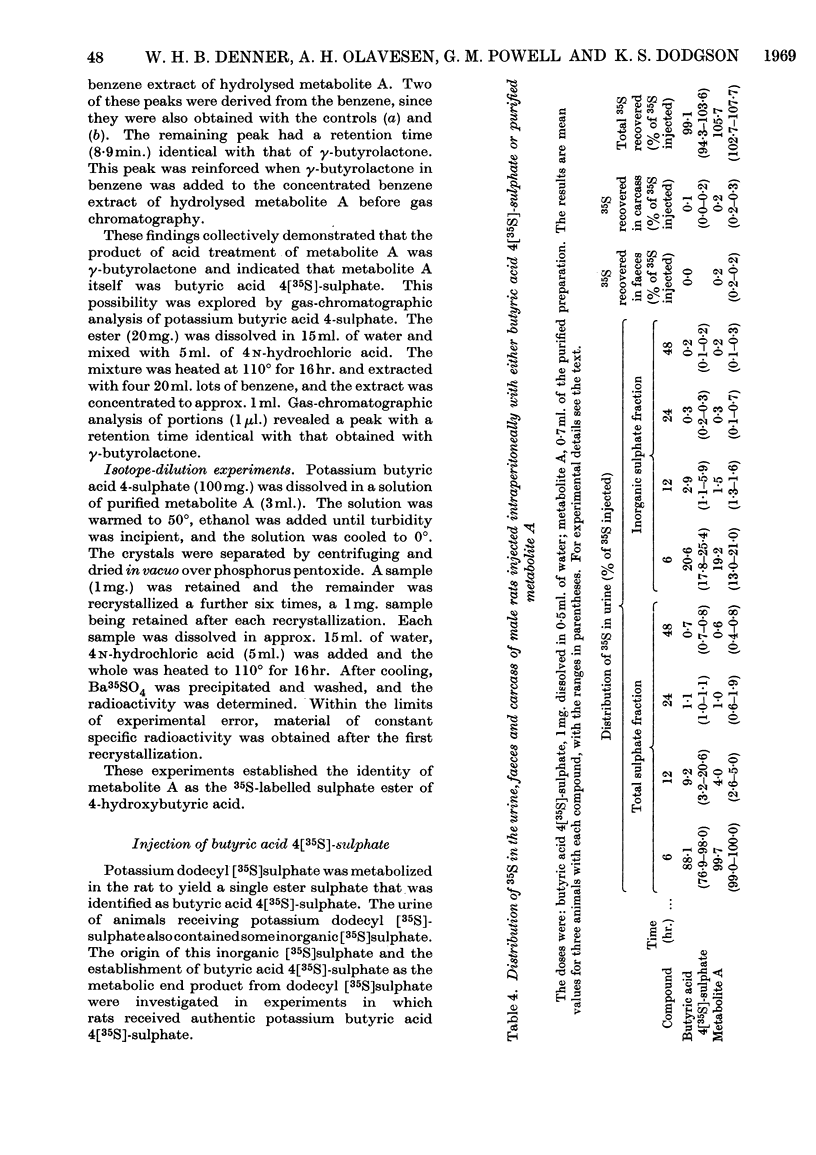
The metabolic fate of potassium dodecyl [35S]sulphate was studied in rats. Intraperitoneal and oral administration of the ester into free-ranging animals were followed by the excretion of the bulk of the radioactivity in the urine within 12hr., approximately 17% being eliminated as inorganic [35S]sulphate. Similar results were obtained in experiments in which potassium dodecyl [35S]sulphate was injected intravenously into anaesthetized rats with bile-duct and ureter cannulae. Analysis of urinary radioactivity revealed the presence of a new ester sulphate (metabolite A). This metabolite was isolated, purified and subsequently identified as the sulphate ester of 4-hydroxybutyric acid by paper, thin-layer and gas chromatography, by paper electrophoresis and by comparison of its properties with those of authentic butyric acid 4-sulphate. The identity of the metabolite was confirmed by isotope-dilution experiments. When either purified metabolite A or authentic potassium butyric acid 4[35S]-sulphate was administered to free-ranging rats the bulk of the radioactivity was eliminated unchanged in the urine within 12hr., approx. 20% of the dose appearing as inorganic [35S]sulphate. Whole-body radioautography and isolated-liver-perfusion experiments implicated the liver as the major site of metabolism of potassium dodecyl [35S]sulphate. It is suggested that butyric acid 4-sulphate probably arises by ω-oxidation of dodecyl sulphate to a fatty acid-like compound, which is then degraded by β-oxidation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASCORBI H. F., RUDO F. G., LU G. G. ACUTE TOXICITY OF INTRAVENOUS SODIUM LAURYL SULFATE. J Pharm Sci. 1963 Aug;52:803–805. doi: 10.1002/jps.2600520822. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., POWELL G. M., ROSE F. A., TUDBALL N. Observations on the metabolism of tyrosine O[35S]-sulphate in the rat. Biochem J. 1961 May;79:209–213. doi: 10.1042/bj0790209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den H. The biological oxidation of 2,2-dimethyloctanoic acid. Biochim Biophys Acta. 1965 Jun 1;98(3):462–469. doi: 10.1016/0005-2760(65)90142-6. [DOI] [PubMed] [Google Scholar]
- Flynn T. G., Dodgson K. S., Powell G. M., Rose F. A. The metabolism of dipotassium 2-hydroxy-5-nitrophenyl [S]sulphate, a substrate for lysosomal arylsulphatases A and B. Biochem J. 1967 Dec;105(3):1003–1012. doi: 10.1042/bj1051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIARMAN N. J., ROTH R. H. DIFFERENTIAL ESTIMATION OF GAMMA-BUTYROLACTONE AND GAMMA-HYDROXYBUTYRIC ACID IN RAT BLOOD AND BRAIN. Science. 1964 Aug 7;145(3632):583–584. doi: 10.1126/science.145.3632.583. [DOI] [PubMed] [Google Scholar]
- JONES J. G., DODGSON K. S. BIOSYNTHESIS OF L-TYROSINE O-SULPHATE FROM THE METHYL AND ETHYL ESTERS OF L-TYROSINE. Biochem J. 1965 Feb;94:331–336. doi: 10.1042/bj0940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWBART M. L., SCHNEIDER J. J. Enzymatic synthesis of steroid sulfates. J Biol Chem. 1956 Oct;222(2):787–794. [PubMed] [Google Scholar]
- PREISS B., BLOCH K. OMEGA-OXIDATION OF LONG CHAIN FATTY ACIDS IN RAT LIVER. J Biol Chem. 1964 Jan;239:85–88. [PubMed] [Google Scholar]
- Powell G. M., Curtis C. G., Dodgson K. S. The distribution of 35S-labelled sulphuric acid esters administered to mice and rats. Biochem Pharmacol. 1967 Oct;16(10):1997–2001. doi: 10.1016/0006-2952(67)90311-5. [DOI] [PubMed] [Google Scholar]
- Robbins K. C. In vitro enzymic omega oxidation of medium-chain fatty acids in mammalian tissue. Arch Biochem Biophys. 1968 Mar 11;123(3):531–538. doi: 10.1016/0003-9861(68)90174-4. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI K., SHIMAZONO N. Studies on omega-oxidation of fatty acids in vitro. I. Overall reaction and intermediate. Biochim Biophys Acta. 1963 Apr 23;70:132–142. doi: 10.1016/0006-3002(63)90733-9. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Brown V. K., Ferrigan L. W., Pickering R. G., Williams D. A. Toxicity os sodium lauryl sulphate, sodium lauryl ethoxysulphate and corresponding surfactants derived from synthetic alcohols. Food Cosmet Toxicol. 1967 Dec;5(6):763–769. doi: 10.1016/s0015-6264(67)83275-9. [DOI] [PubMed] [Google Scholar]
- Young L., Edson M., McCarter J. A. The measurement of radioactive sulphur (S) in biological material. Biochem J. 1949;44(2):179–185. [PMC free article] [PubMed] [Google Scholar]


