Abstract
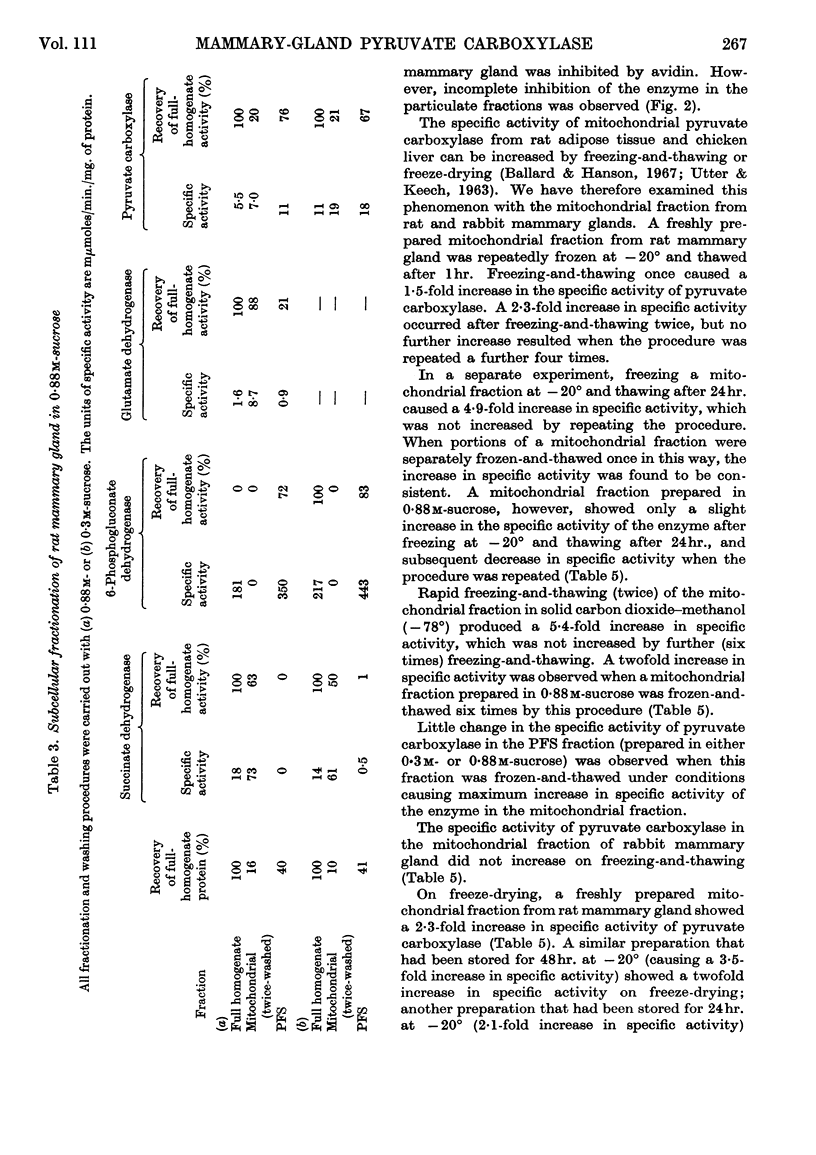
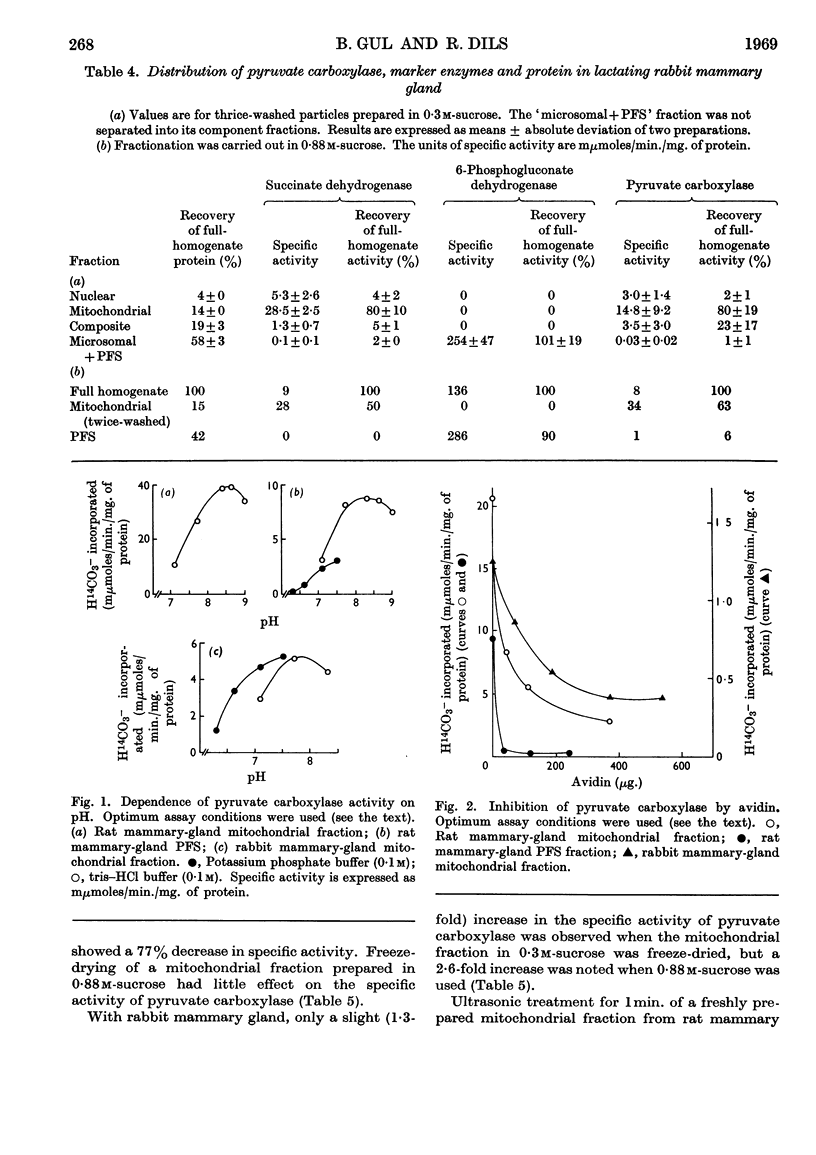
1. Pyruvate carboxylase [pyruvate–carbon dioxide ligase (ADP), EC 6.4.1.1] was found in cell-free preparations of lactating rat and rabbit mammary glands, and optimum assay conditions for this enzyme were determined. 2. Subcellular-fractionation studies with marker enzymes showed pyruvate carboxylase to be distributed between the mitochondrial and soluble fractions of lactating rat mammary gland. Evidence is presented that the soluble enzyme is not an artifact due to mitochondrial damage. 3. In contrast, pyruvate carboxylase in lactating rabbit mammary gland is confined to the mitochondrial fraction. 4. The final product of pyruvate carboxylase action in the mitochondrial and particle-free supernatant fractions of lactating rat mammary gland was shown to be citrate. 5. The effects of freeze-drying, ultrasonic treatment and freezing-and-thawing on the specific activity of mitochondrial pyruvate carboxylase were investigated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSCH H., HURLBERT R. B., POTTER V. R. Anion exchange chromatography of acids of the citric acid cycle. J Biol Chem. 1952 May;196(2):717–727. [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. The citrate cleavage pathway and lipogenesis in rat adipose tissue: replenishment of oxaloacetate. J Lipid Res. 1967 Mar;8(2):73–79. [PubMed] [Google Scholar]
- Easter D. J., Dils R. Fatty acid biosynthesis. IV. Properties of acetyl-CoA carboxylase in lactating-rabbit mammary gland. Biochim Biophys Acta. 1968 Jul 1;152(4):653–668. doi: 10.1016/0005-2760(68)90112-4. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- GAILIUSIS J., RINNE R. W., BENEDICT C. R. PYUVATE-OXALOACETATE EXCHANGE REACTION IN BAKER'S YEAST. Biochim Biophys Acta. 1964 Dec 23;92:595–601. doi: 10.1016/0926-6569(64)90019-7. [DOI] [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- Gul B., Dils R. Pyruvate carboxylase in lactating rat and rabbit mammary gland. Biochem J. 1968 Sep;109(3):26P–27P. doi: 10.1042/bj1090026pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning H. V., Stumpf B., Ohly B., Seubert W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem Z. 1966 Apr 27;344(3):274–288. [PubMed] [Google Scholar]
- Hultin H. O., Westort C., Southard J. H. Adsorption of lactate dehydrogenase to the particulate fraction of homogenized skeletal muscle. Nature. 1966 Aug 20;211(5051):853–854. doi: 10.1038/211853a0. [DOI] [PubMed] [Google Scholar]
- JONES E. A., GUTFREUND H. The control of some oxidative pathways in guinea-pig mammary-gland mitochondria. Biochem J. 1961 Jun;79:608–614. doi: 10.1042/bj0790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. II. PROPERTIES. J Biol Chem. 1963 Aug;238:2609–2614. [PubMed] [Google Scholar]
- Ling A. M., Keech D. B. Pyruvate carboxylase from sheep kidney. I. Purification and some properties of the enzyme. Enzymologia. 1966 Jun 30;30(6):367–380. [PubMed] [Google Scholar]
- Mehlman M. A., Walter P., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. VII. The synthesis of precursors for gluconeogenesis from pyruvate and bicarbonate by rat kidney mitochondria. J Biol Chem. 1967 Oct 25;242(20):4594–4602. [PubMed] [Google Scholar]
- Smith S., Easter D. J., Dils R. Fatty acid biosynthesis. 3. Intracellular site of enzymes in lactating-rabbit mammary gland. Biochim Biophys Acta. 1966 Dec 7;125(3):445–455. [PubMed] [Google Scholar]
- Spencer A. F., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Citrate cleavage in obesity and lactation. Biochem J. 1966 Jun;99(3):760–765. doi: 10.1042/bj0990760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tame M. J., Kils R. Fatty acid synthesis in intestinal mucosa of guinea pig. Biochem J. 1967 Nov;105(2):709–716. doi: 10.1042/bj1050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION. J Biol Chem. 1963 Aug;238:2603–2608. [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Paetkau V., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem. 1966 Jun 10;241(11):2523–2532. [PubMed] [Google Scholar]
- ZIEGLER D. M., LINNANE A. W., GREEN D. E., DASS C. M., RIS H. Studies on the electron transport system. XI. Correlation of the morphology and enzymic properties of mitochondrial and sub-mitochondrial particles. Biochim Biophys Acta. 1958 Jun;28(3):524–538. doi: 10.1016/0006-3002(58)90515-8. [DOI] [PubMed] [Google Scholar]
- ZIEGLER D. M., LINNANE A. W. Studies on the electron transport system. XIII. Mitochondrial structure and dehydrogenase activity in isolated mitochondria. Biochim Biophys Acta. 1958 Oct;30(1):53–63. doi: 10.1016/0006-3002(58)90240-3. [DOI] [PubMed] [Google Scholar]


