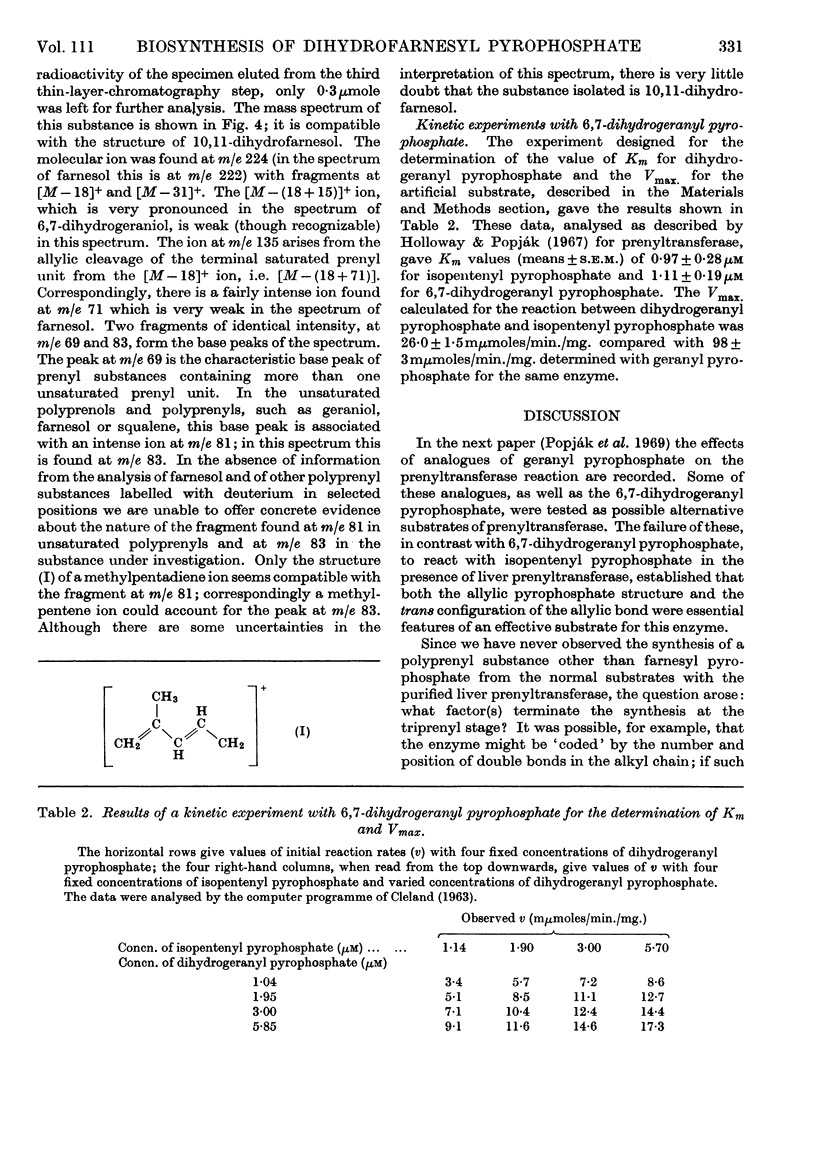
Abstract
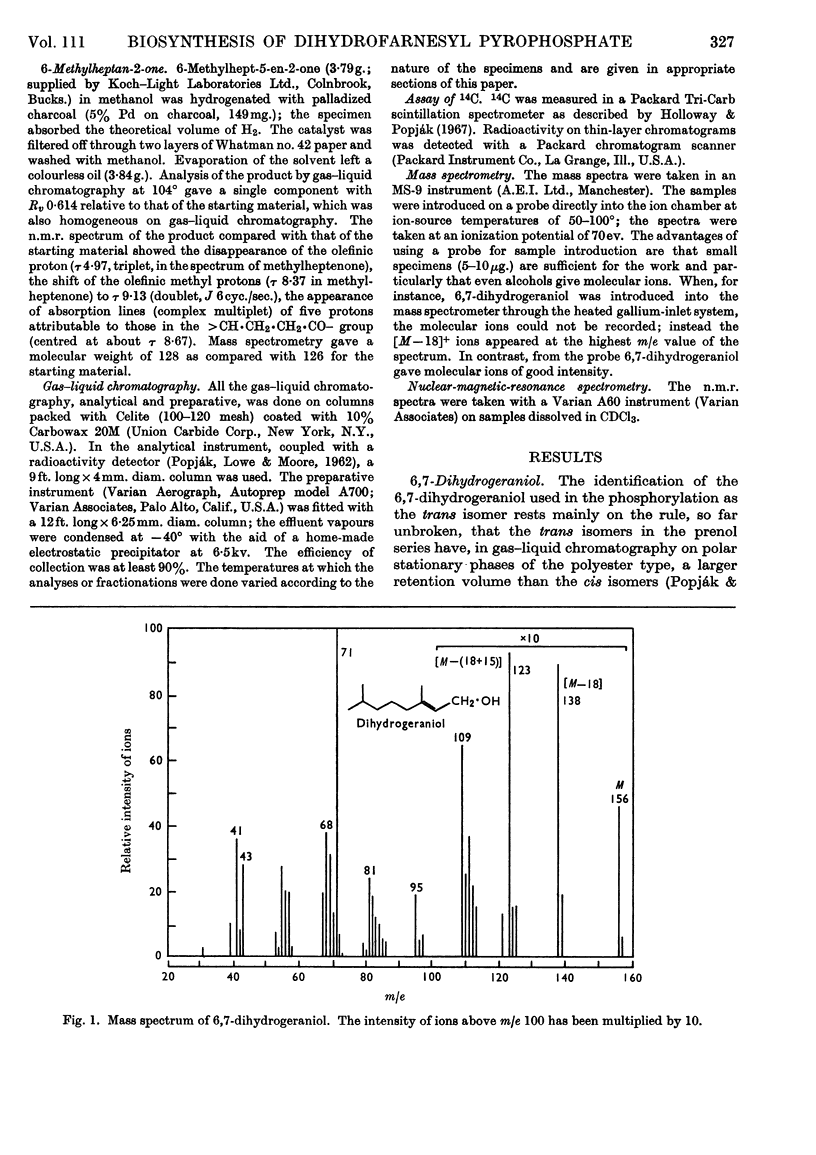
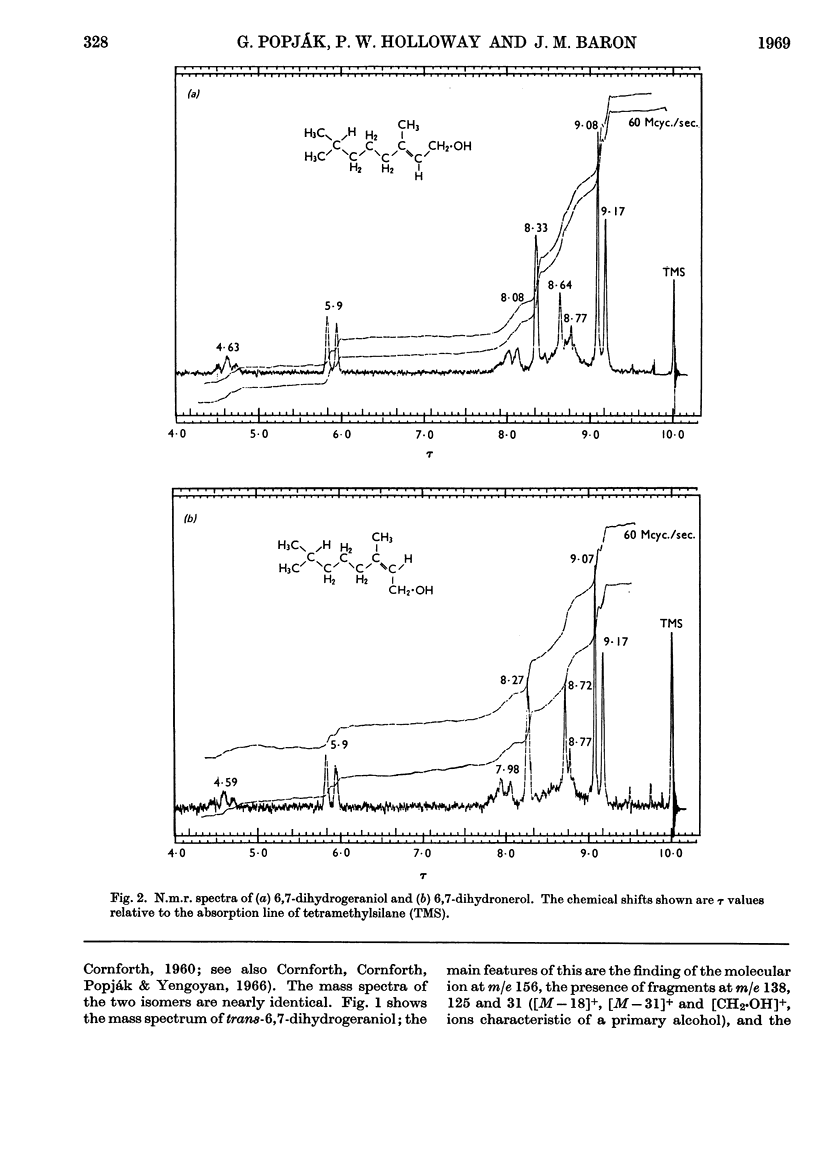
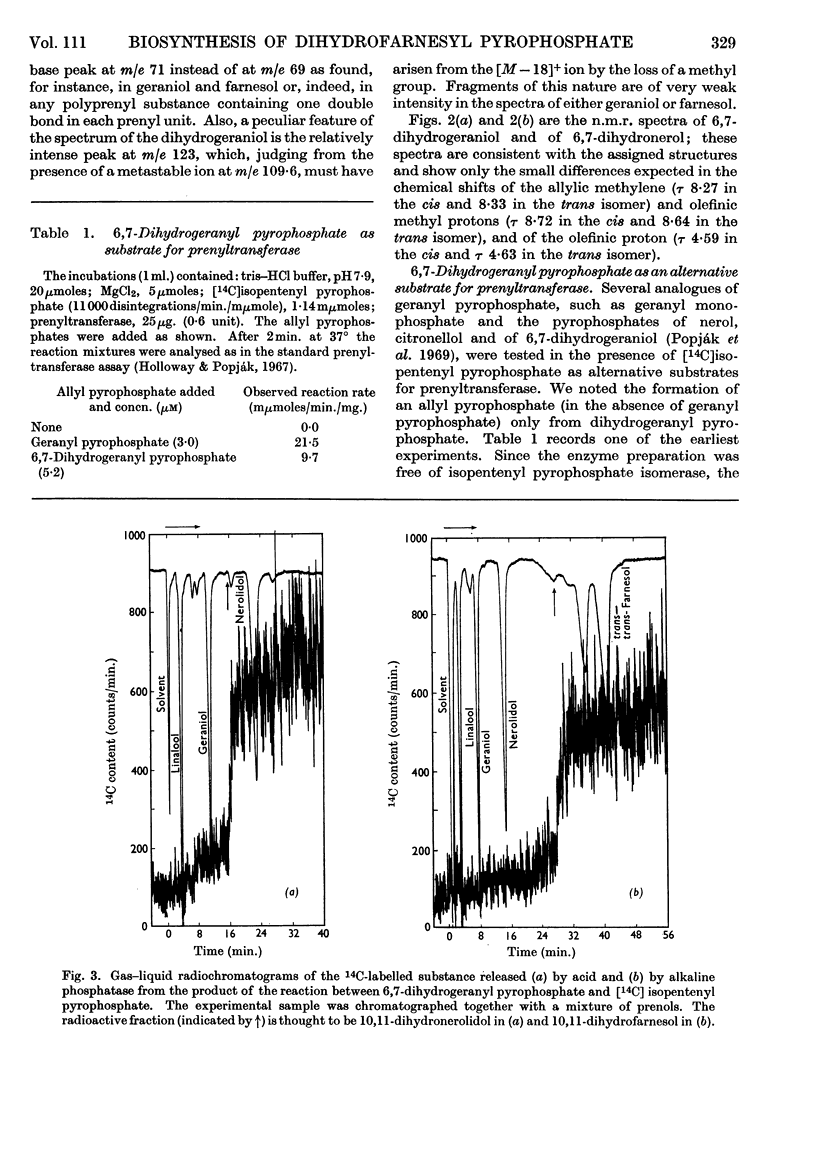
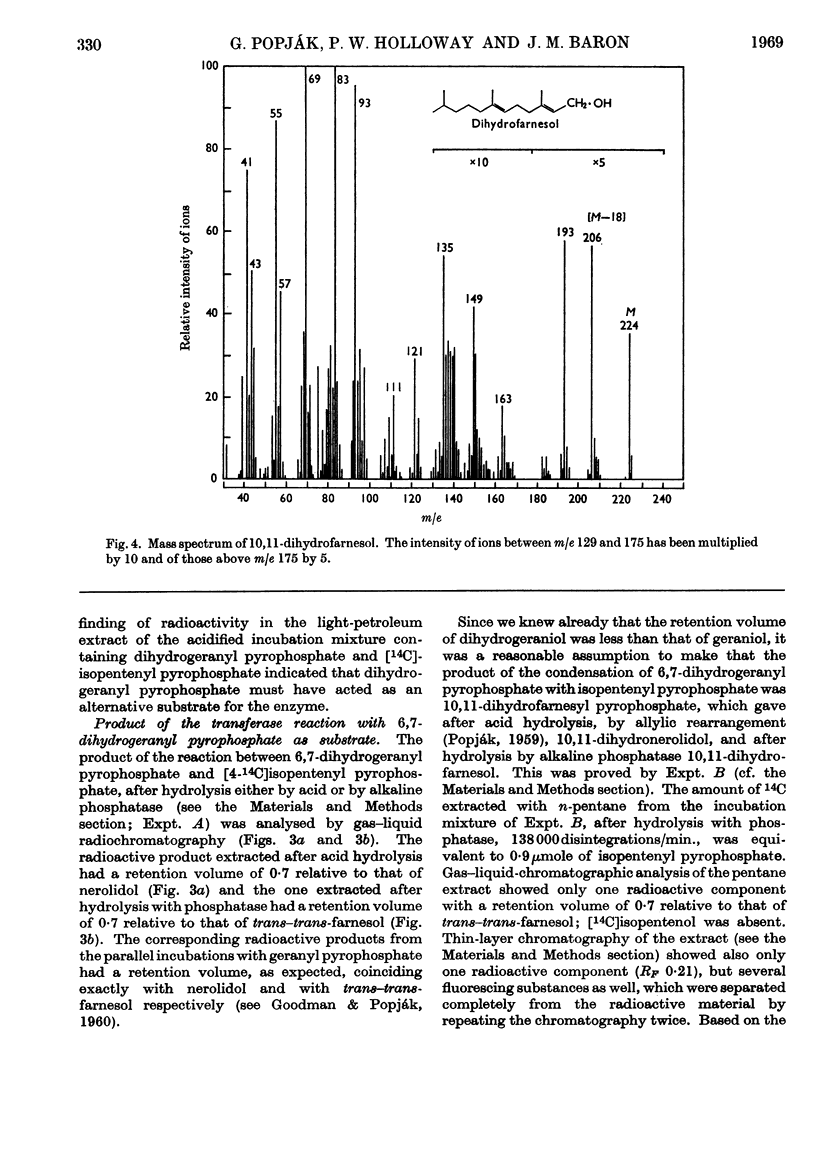
The syntheses of 6,7-dihydrogeraniol and of its pyrophosphate are described. It is shown that this analogue of geranyl pyrophosphate is a substrate for liver prenyltransferase and that the product synthesized by this enzyme from it and isopentenyl pyrophosphate is 10,11-dihydrofarnesyl pyrophosphate. The Km value for 6,7-dihydrogeranyl pyrophosphate was determined to be 1·11±0·19μm as compared with 4·34±1·71μm for geranyl pyrophosphate. The maximum reaction velocity with the artifical substrate was, however, only about one-fourth of that observed with geranyl pyrophosphate. The binding of isopentenyl pyrophosphate to the enzyme was not affected by the artificial substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Popják G., Yengoyan L. Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate. J Biol Chem. 1966 Sep 10;241(17):3970–3987. [PubMed] [Google Scholar]
- Donninger C., Popják G. An improved synthesis of isopentenyl pyrophosphate. Biochem J. 1967 Nov;105(2):545–547. doi: 10.1042/bj1050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W., Popják G. The purification of 3,3-dimethylallyl- and geranyl-transferase and of isopentenyl pyrophosphate isomerase from pig liver. Biochem J. 1967 Jul;104(1):57–70. doi: 10.1042/bj1040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPJAK G., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R., GOODMAN D. S. Studies on the biosynthesis of cholesterol. XVI. Chemical synthesis of 1-H2-3-2-C-14- and 1-D2-2-C-14-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962 Jan;237:56–61. [PubMed] [Google Scholar]
- Popják G., Holloway P. W., Bond R. P., Roberts M. Analogues of geranyl pyrophosphate as inhibitors of prenyltransferase. Biochem J. 1969 Feb;111(3):333–343. doi: 10.1042/bj1110333. [DOI] [PMC free article] [PubMed] [Google Scholar]


