Abstract
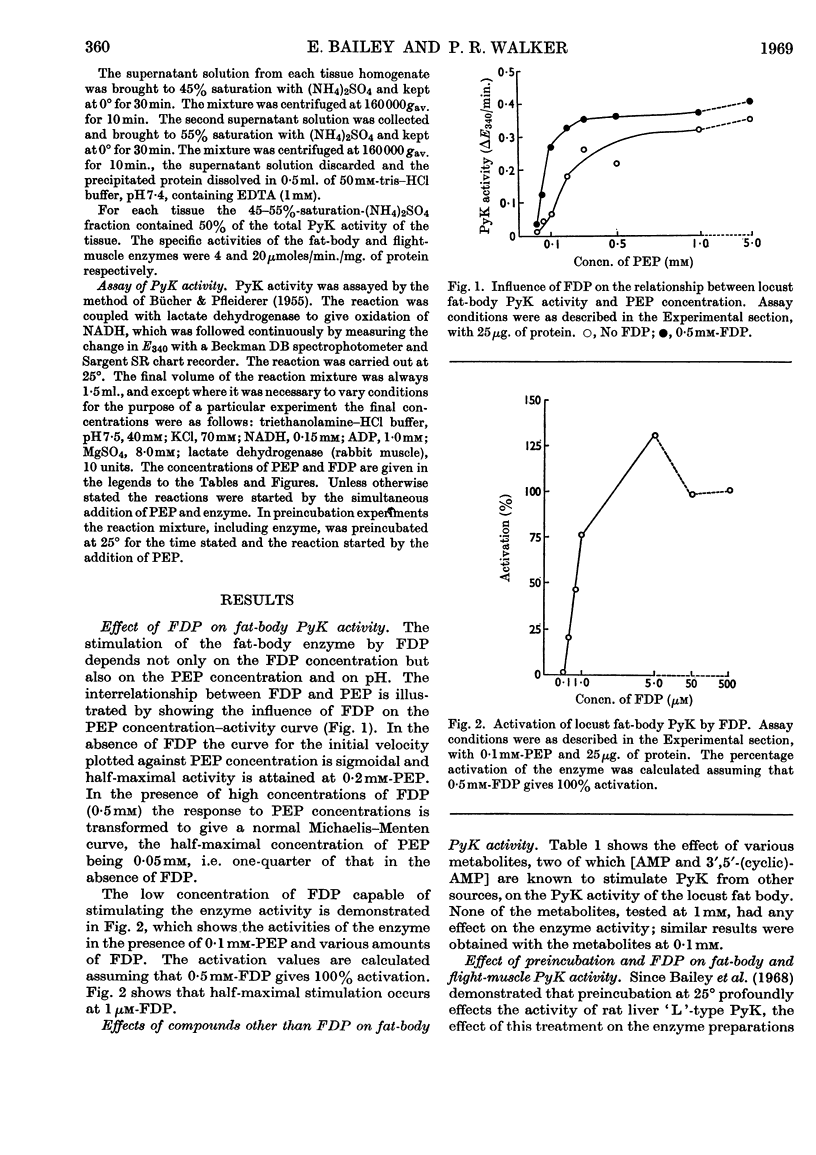
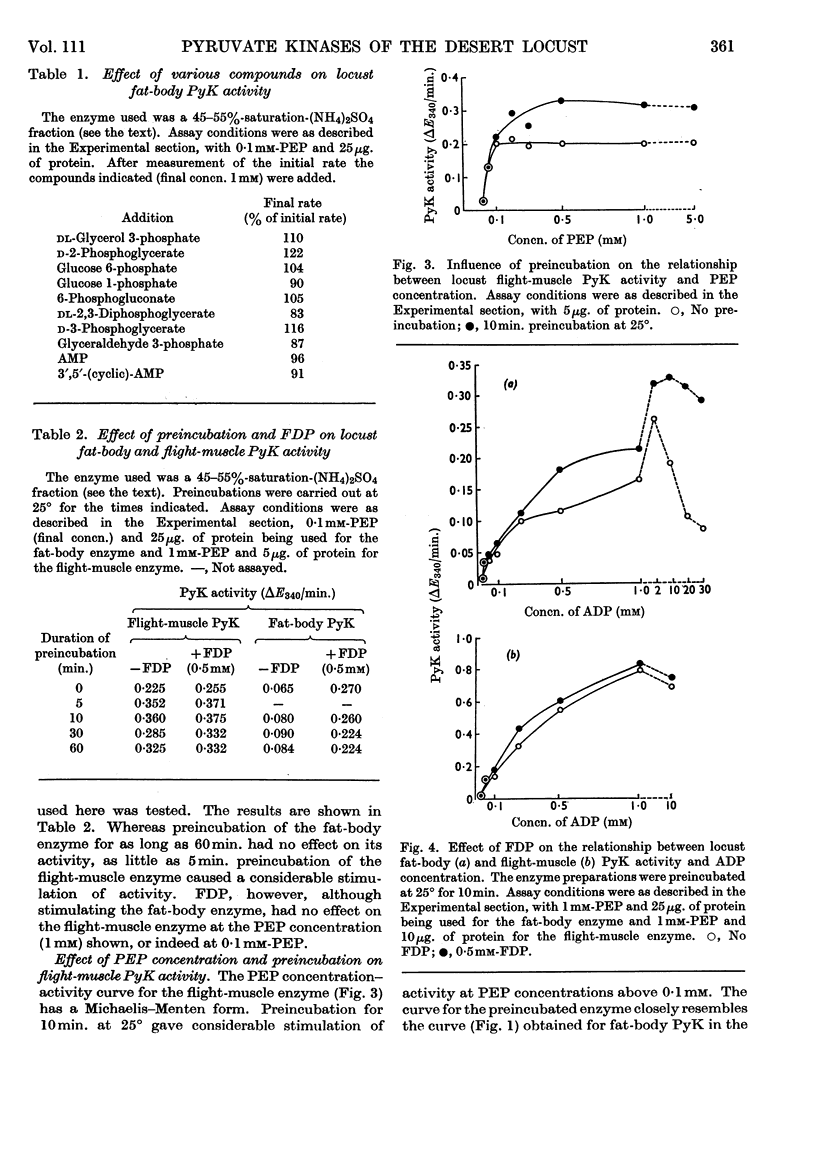
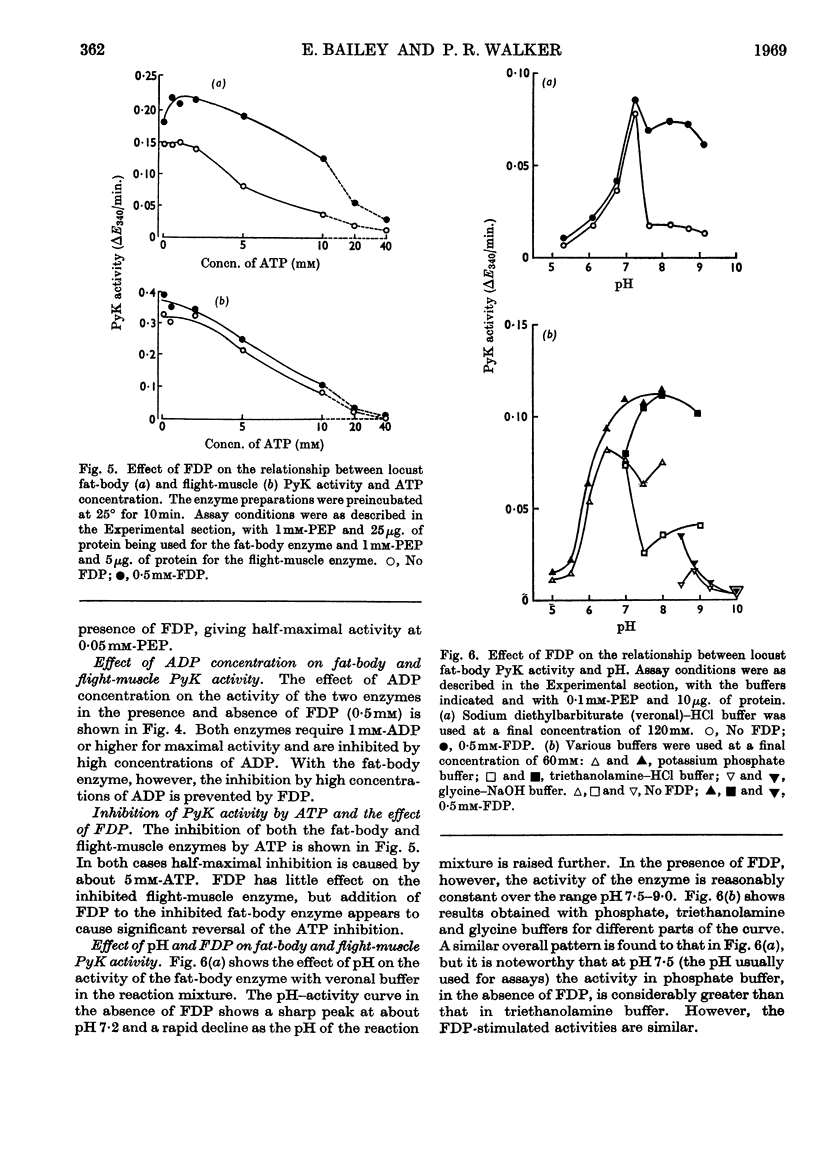
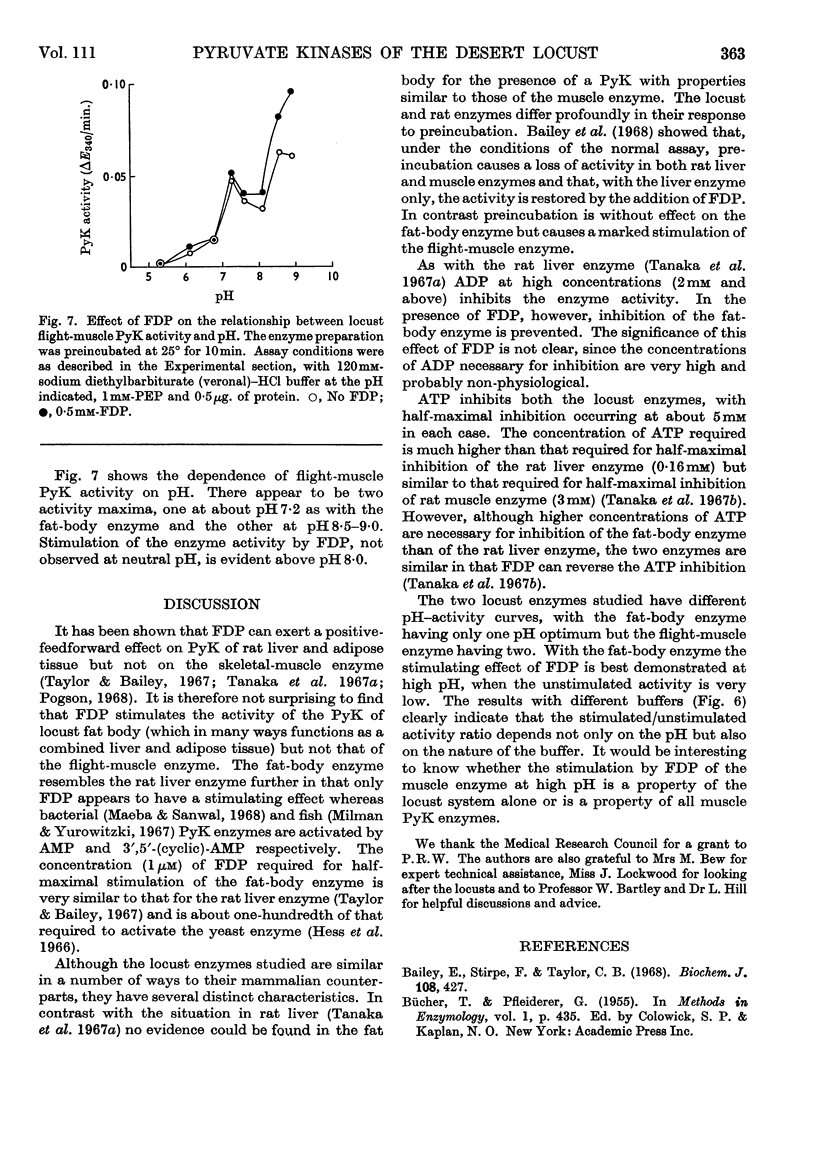
1. The pyruvate kinases of the desert locust fat body and flight muscle were partially purified by ammonium sulphate fractionation. 2. The fat-body enzyme is allosterically activated by very low (1μm) concentrations of fructose 1,6-diphosphate, whereas the flight-muscle enzyme is unaffected by this metabolite at physiological pH. 3. Flight-muscle pyruvate kinase is activated by preincubation at 25° for 5min., whereas the fat-body enzyme is unaffected by such treatment. 4. Both enzymes require 1–2mm-ADP for maximal activity and are inhibited at higher concentrations. With the fat-body enzyme inhibition by ADP is prevented by the presence of fructose 1,6-diphosphate. 5. Both enzymes are inhibited by ATP, half-maximal inhibition occurring at about 5mm-ATP. With the fat-body enzyme ATP inhibition can be reversed by fructose 1,6-diphosphate. 6. The fat-body enzyme exhibits maximal activity at about pH7·2 and the activity decreases rapidly above this pH. This inactivation at high pH is not observed in the presence of fructose 1,6-diphosphate, i.e. maximum stimulating effects of fructose 1,6-diphosphate are observed at high pH. The flight-muscle enzyme exhibits two optima, one at about pH7·2 as with the fat-body enzyme and the other at about pH8·5. Stimulation of the enzyme activity by fructose 1,6-diphosphate was observed at pH8·5 and above.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey E., Stirpe F., Taylor C. B. Regulation of rat liver pyruvate kinase. The effect of preincubation, pH, copper ions, fructose 1,6-diphosphate and dietary changes on enzyme activity. Biochem J. 1968 Jul;108(3):427–436. doi: 10.1042/bj1080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem. 1968 Jan 25;243(2):448–450. [PubMed] [Google Scholar]
- Milman L. S., Yurowitzki YuG Fructose 1,6-diphosphate and 3',5'-cyclic AMP as positive effectors of pyruvate kinase in developing embryos. Biochim Biophys Acta. 1967 Sep 12;146(1):301–304. doi: 10.1016/0005-2744(67)90101-5. [DOI] [PubMed] [Google Scholar]
- Passeron S., Jiménez de Asua L., Carminatti H. Fructose 1,6-diphosphate, a reactivator of Cu++-inhibited pyruvate kinase from liver. Biochem Biophys Res Commun. 1967 Apr 7;27(1):33–38. doi: 10.1016/s0006-291x(67)80035-4. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Two interconvertible forms of pyruvate kinase in adipose tissue. Biochem Biophys Res Commun. 1968 Feb 15;30(3):297–302. doi: 10.1016/0006-291x(68)90450-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]


