Abstract
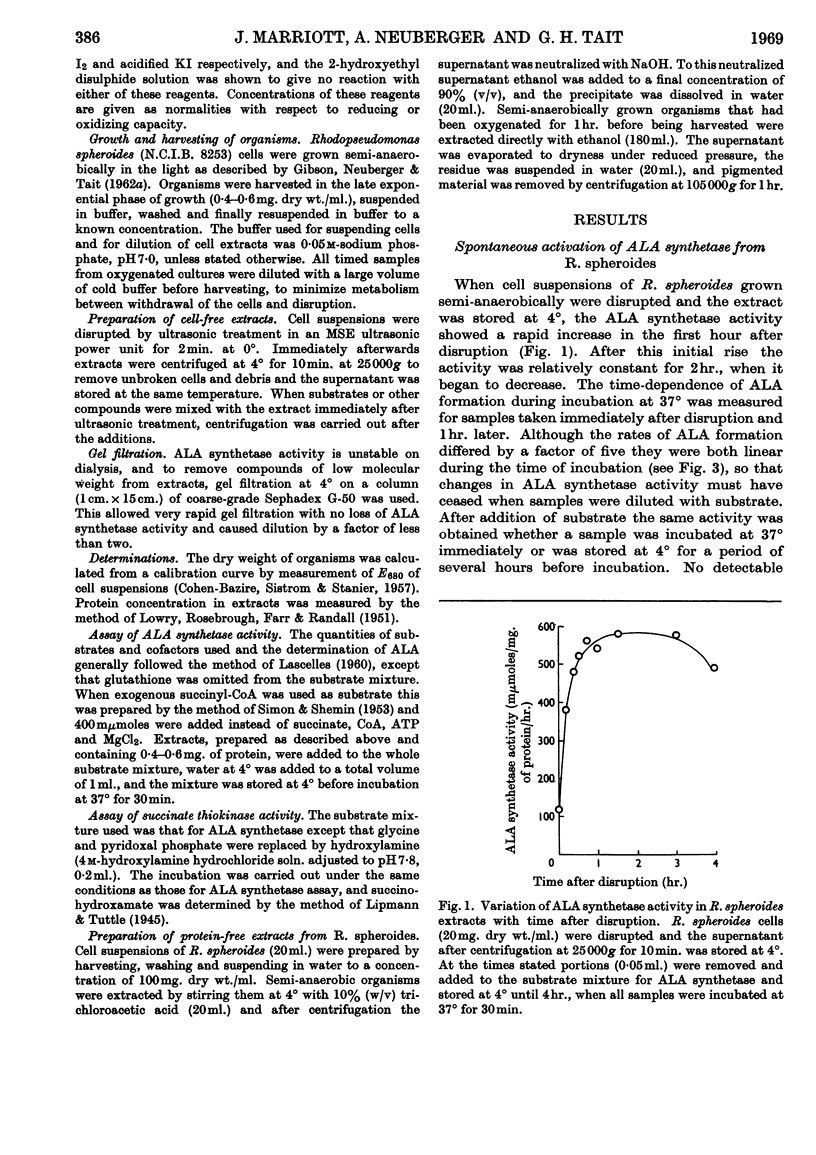
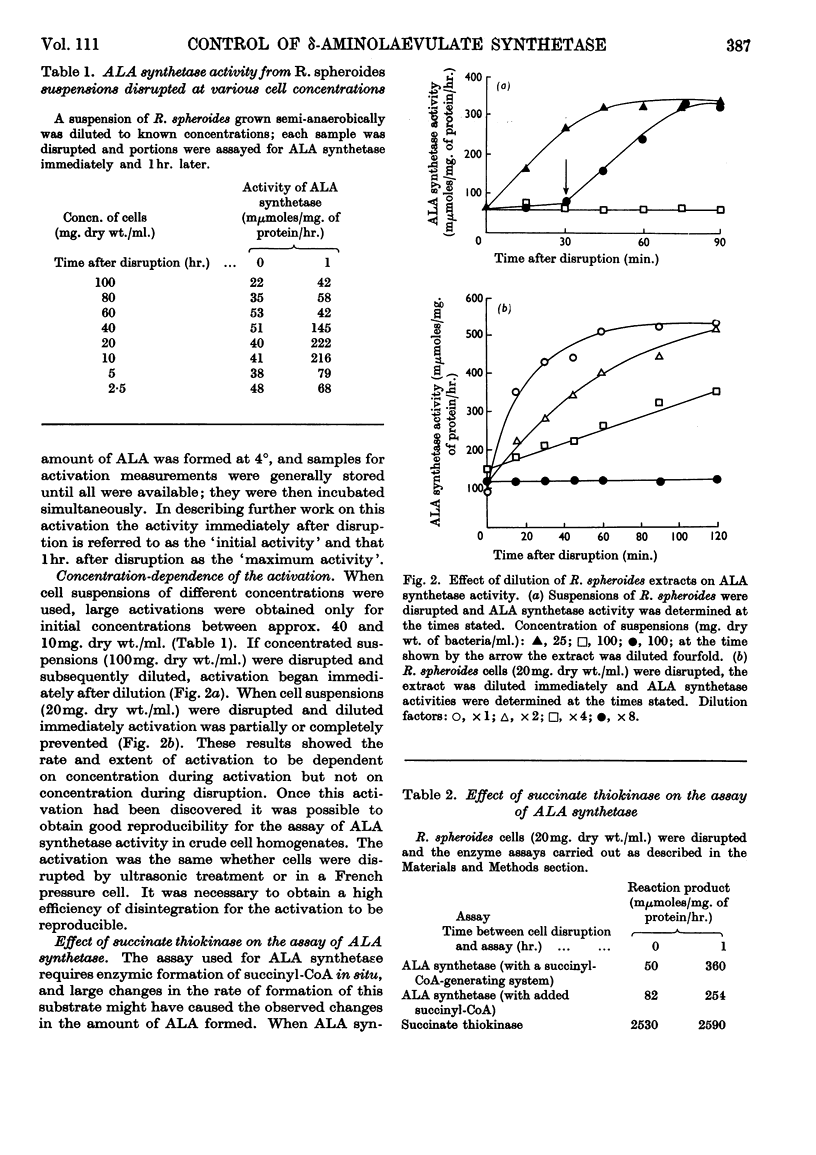
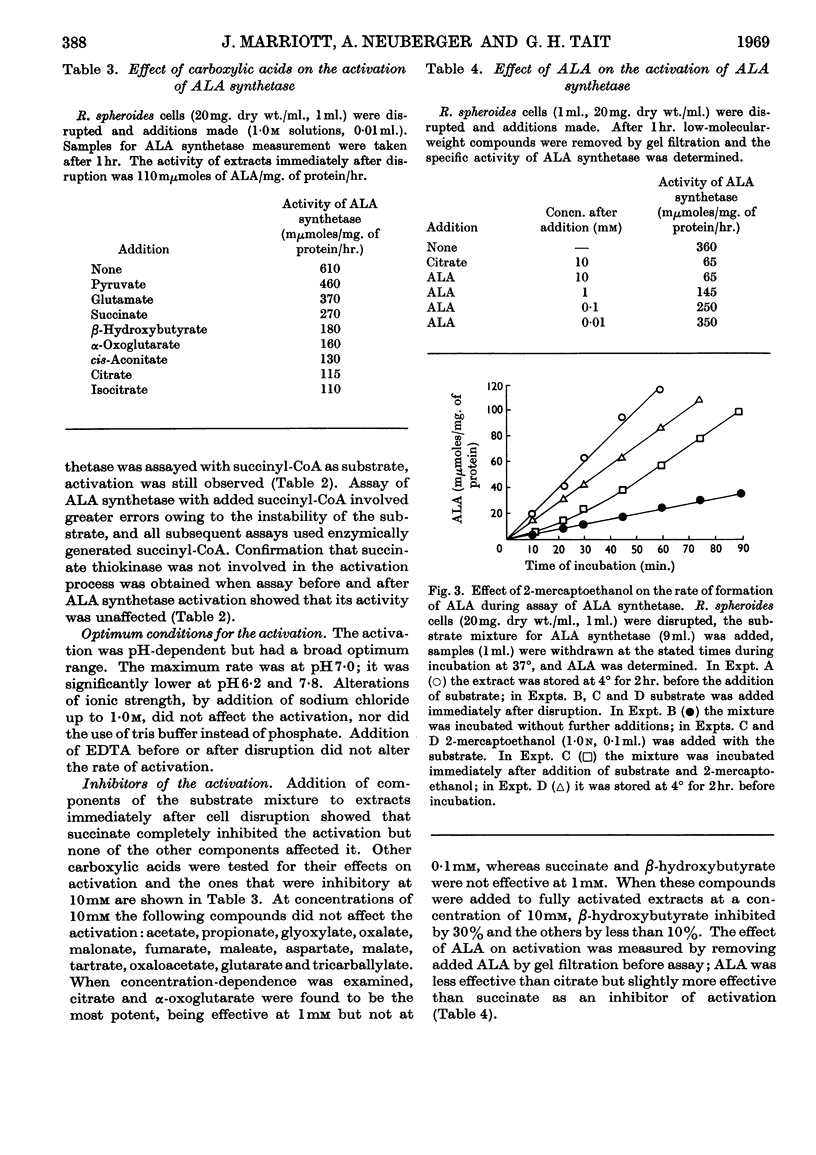
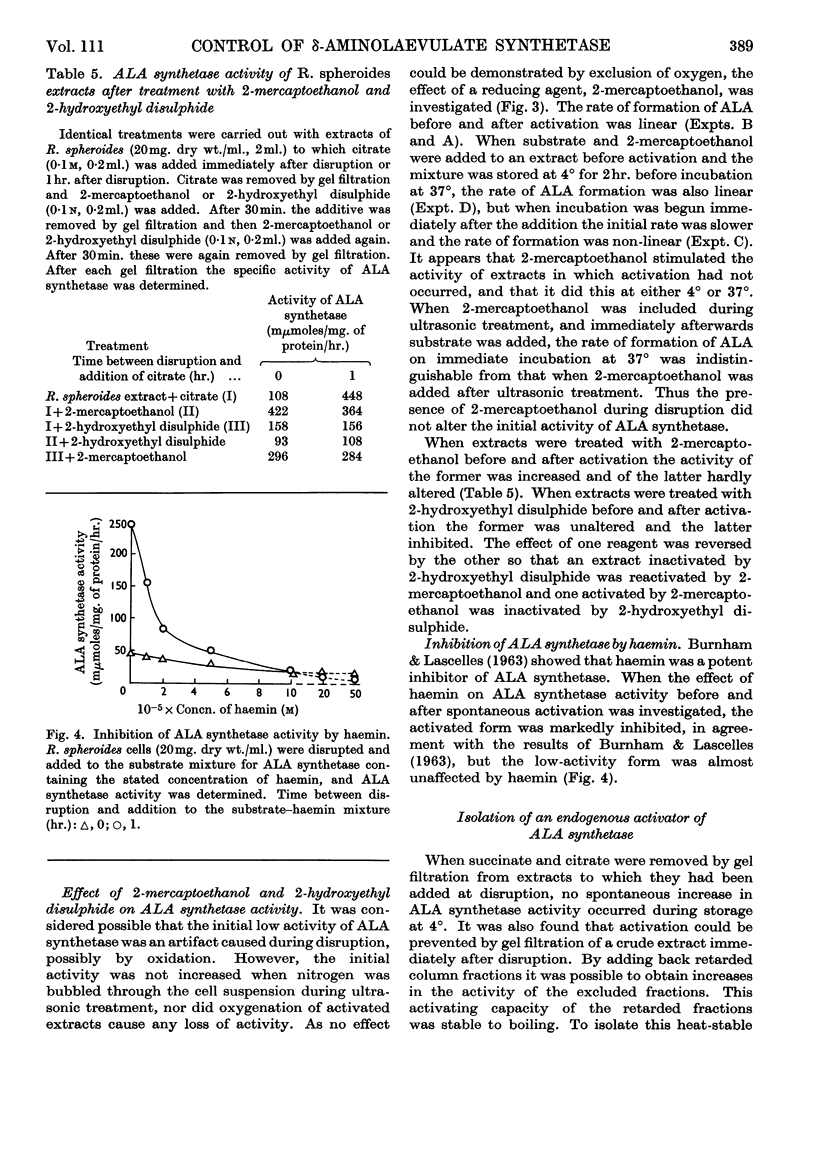
1. δ-Aminolaevulate synthetase from Rhodopseudomonas spheroides grown semi-anaerobically undergoes a spontaneous activation during the first hour after the disruption of cells when homogenates are stored at 4°. 2. After cultures of R. spheroides growing semi-anaerobically are oxygenated no activation of δ-aminolaevulate synthetase occurs in cell extracts. Cessation of activation in extracts is almost complete 10min. after oxygenation of cells has begun. 3. A heat-stable fraction of low molecular weight from semi-anaerobic cells reactivates δ-aminolaevulate synthetase in extracts of oxygenated cells and appears to contain a compound responsible for the spontaneous activation. 4. A heat-stable fraction of low molecular weight from oxygenated cells inhibits the spontaneous activation in extracts of semi-anaerobic cells. 5. The effect of oxygen on the rate of bacteriochlorophyll synthesis in R. spheroides may be mediated through alterations in the concentrations of a low-molecular-weight activator and inhibitor of δ-aminolaevulate synthetase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 1. The effect of growth conditions. Biochem J. 1962 Jun;83:539–549. doi: 10.1042/bj0830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 2. The effects of ethionine and threonine. Biochem J. 1962 Jun;83:550–559. doi: 10.1042/bj0830550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. Adaptation of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Aug 13;171(1022):111–125. doi: 10.1098/rspb.1968.0060. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LESSIE T. G., SISTROM W. R. CONTROL OF PORPHYRIN SYNTHESIS IN RHODOPSEUMOMONAS SPHEROIDES. Biochim Biophys Acta. 1964 May 11;86:250–259. doi: 10.1016/0304-4165(64)90050-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J. The regulation of haem and chlorophyll synthesis. Biochem Soc Symp. 1968;28:49–59. [PubMed] [Google Scholar]
- Leitzmann C., Bernlohr R. W. Threonine dehydratase of Bacillus licheniformis. II. Regulation during development. Biochim Biophys Acta. 1968 Feb 5;151(2):461–472. doi: 10.1016/0005-2744(68)90114-9. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induced biosynthesis of lysine decarboxylase in Bacterium cadaveris. J Gen Microbiol. 1954 Dec;11(3):426–437. doi: 10.1099/00221287-11-3-426. [DOI] [PubMed] [Google Scholar]
- Marriott J. Regulation of porphyrin synthesis. Biochem Soc Symp. 1968;28:61–74. [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]


