Abstract
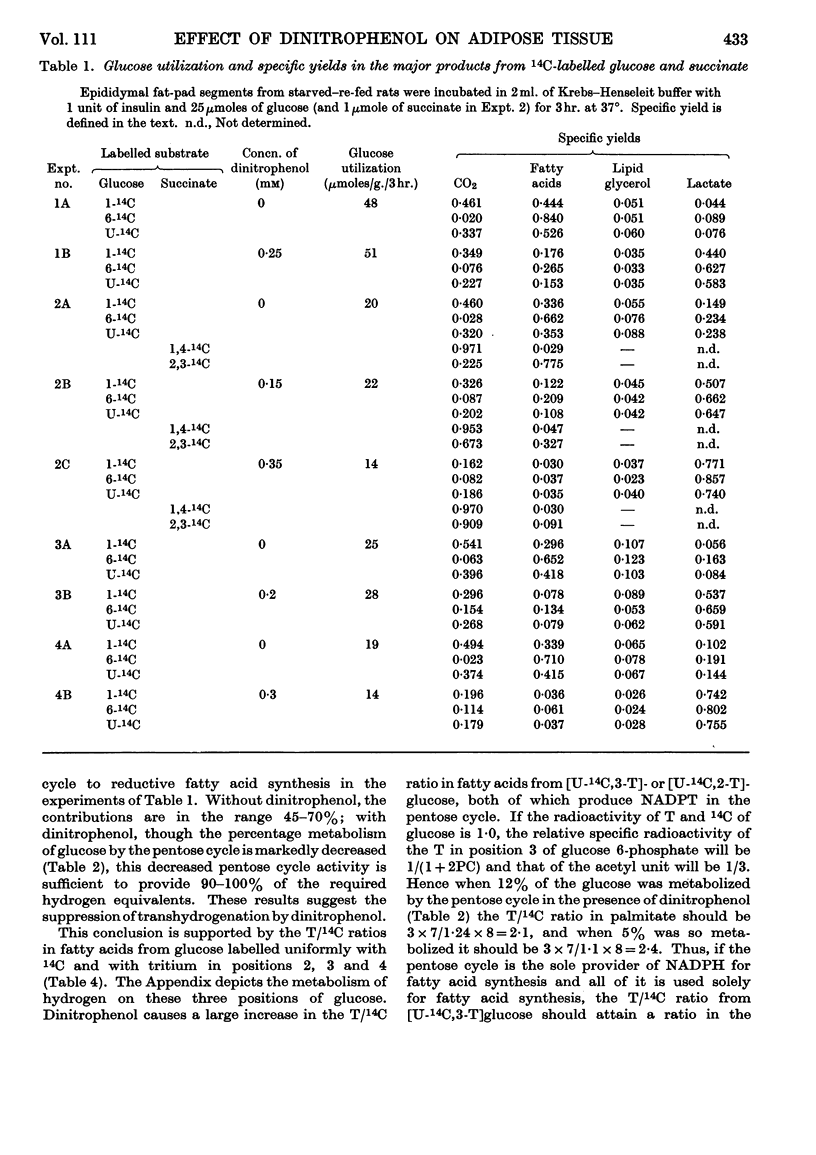
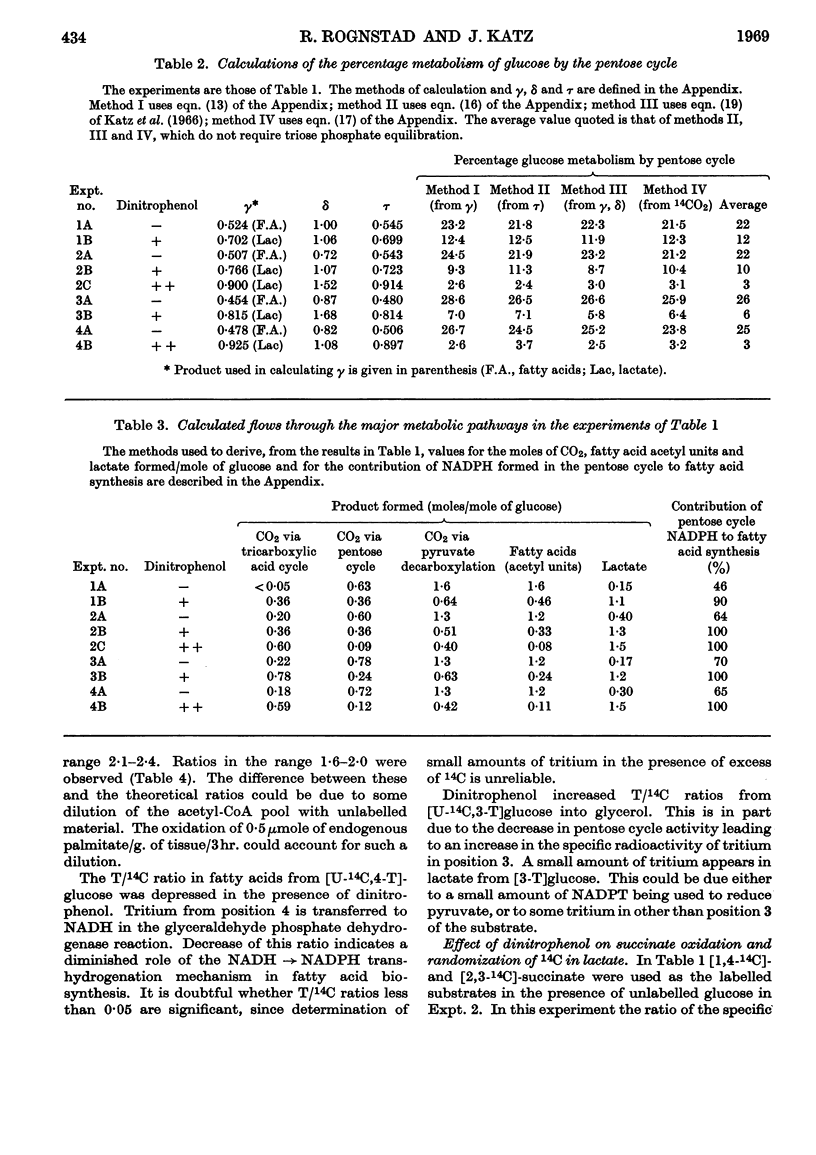
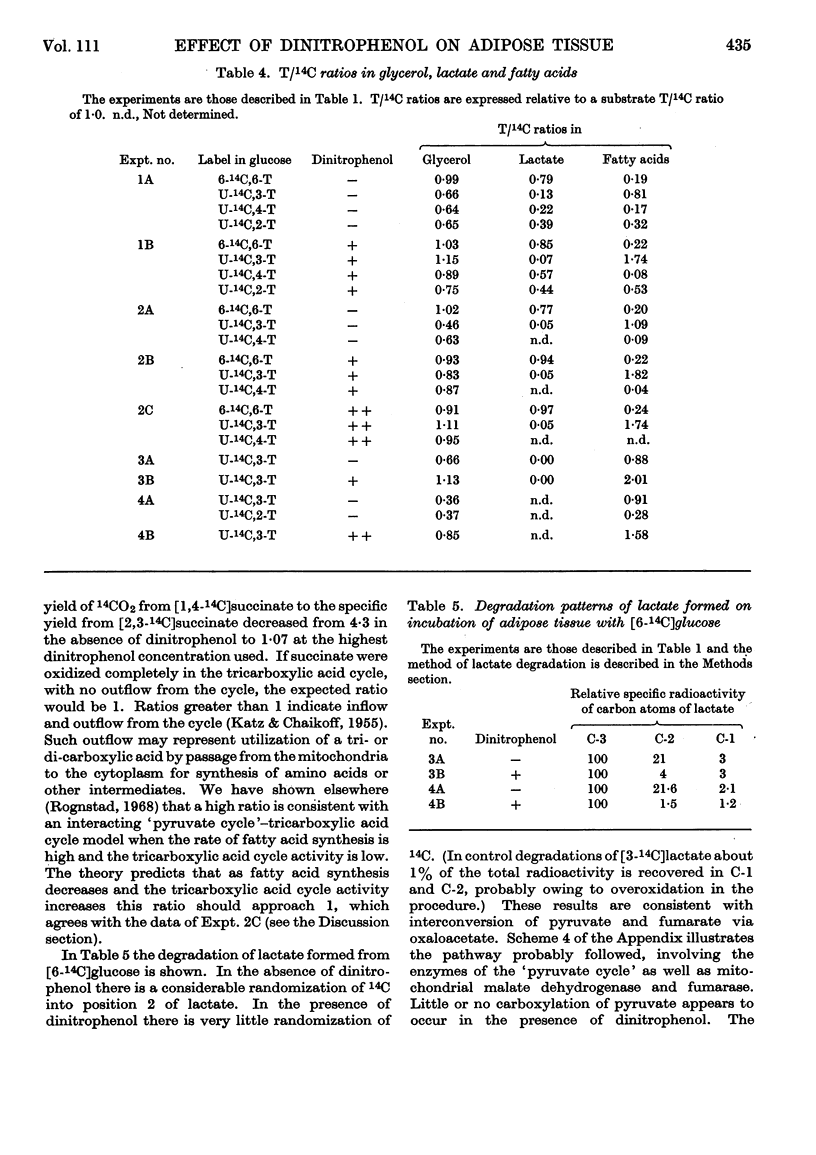
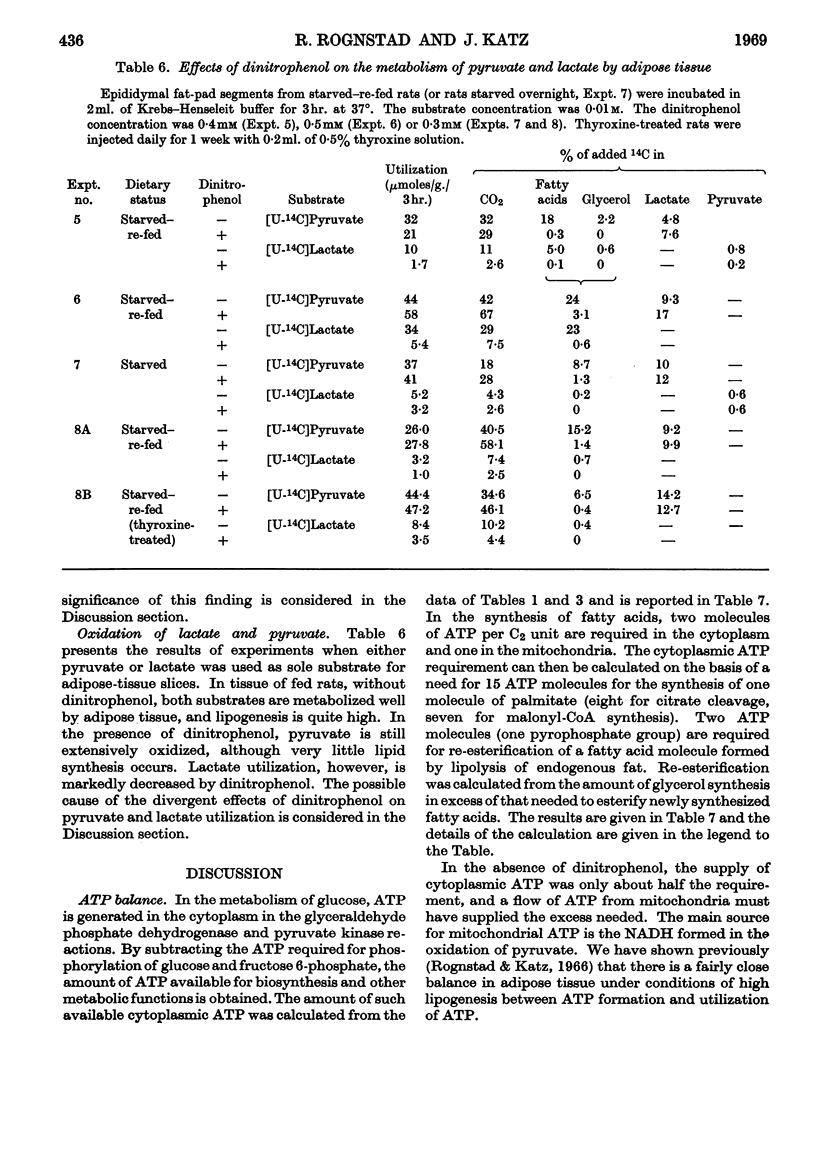
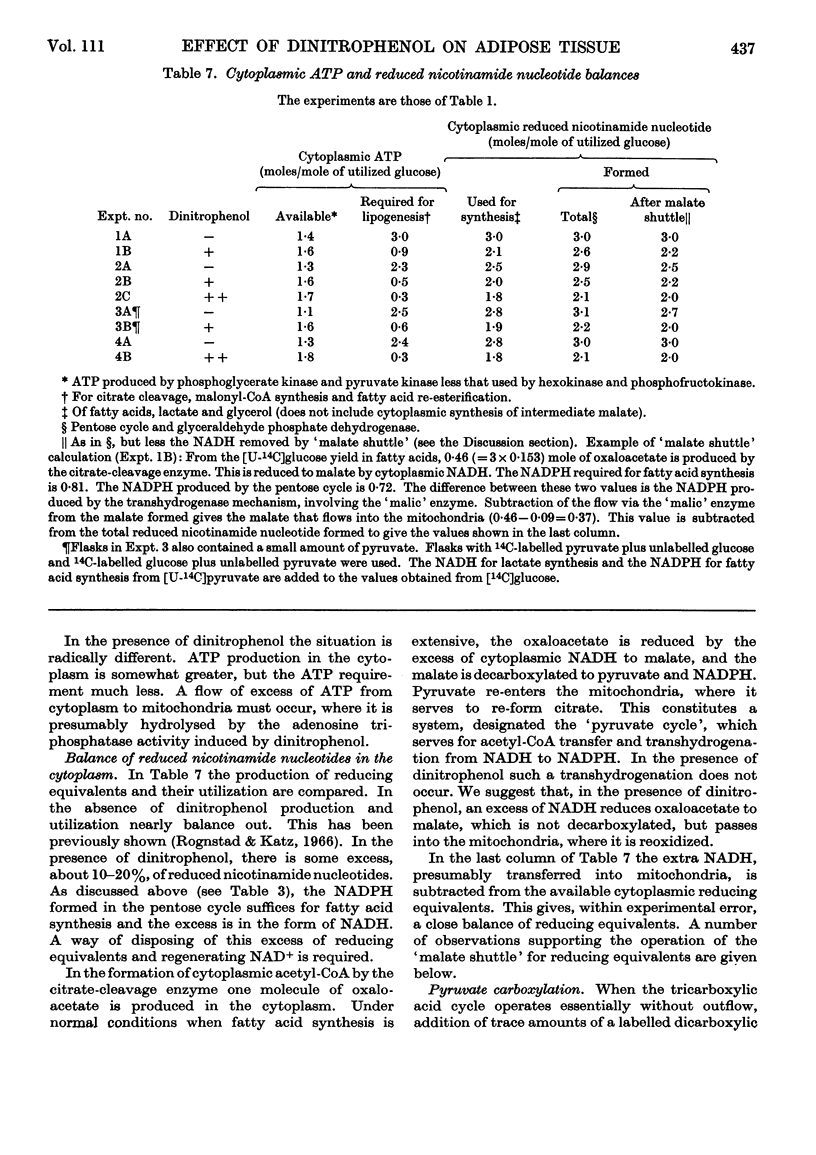
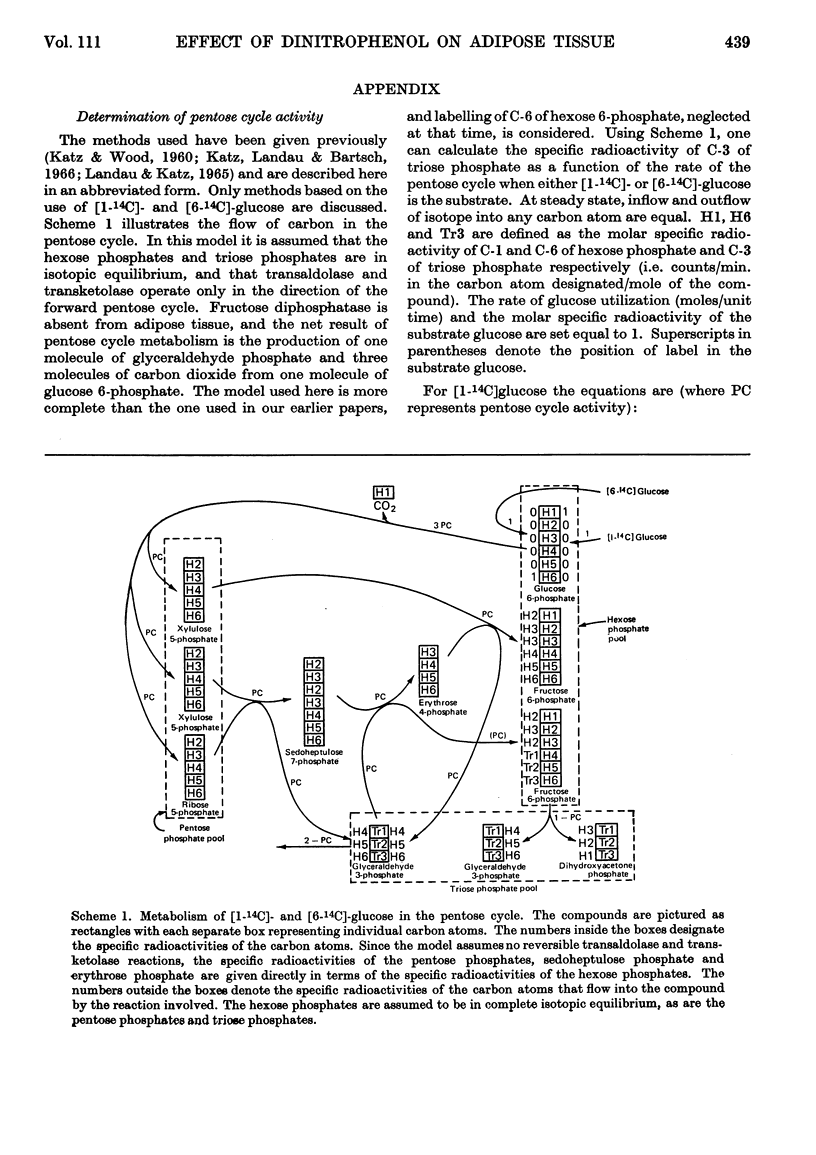
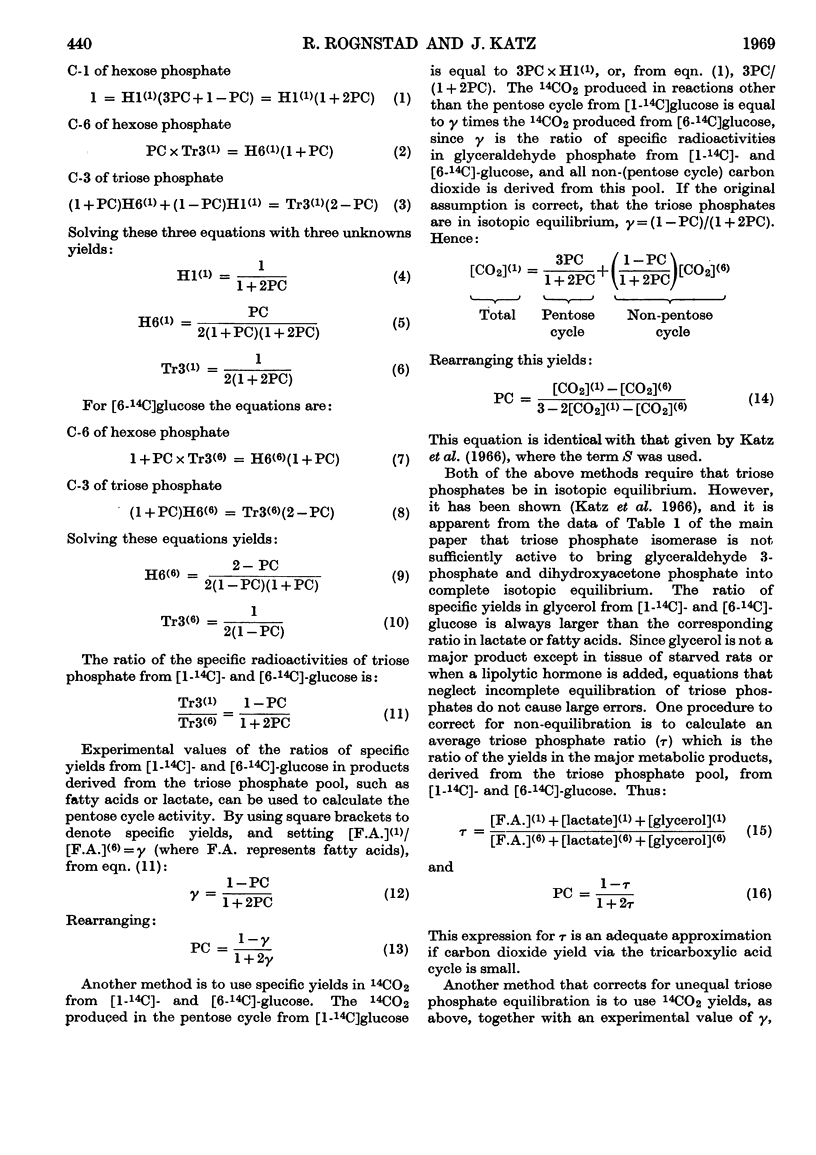
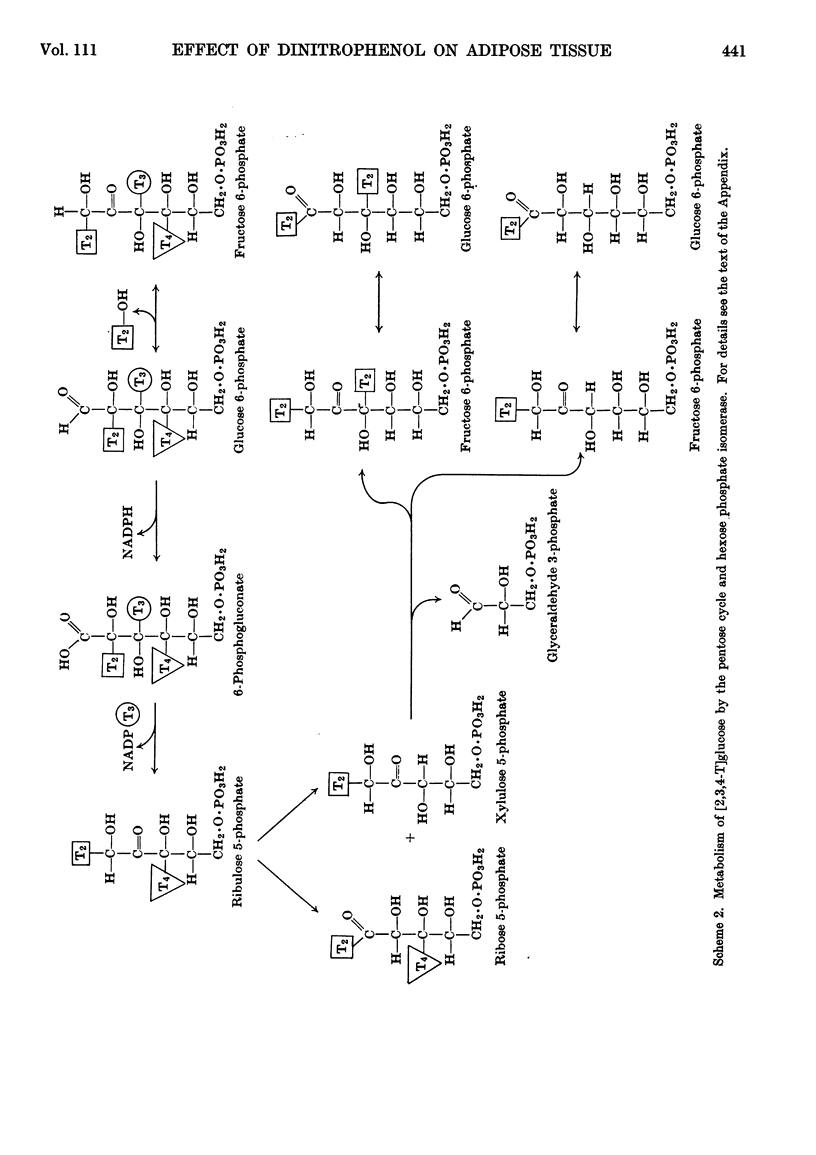
1. The effect of dinitrophenol on the metabolism of glucose labelled with 14C and tritium by epididymal fat-pad segments from fed rats was studied. Dinitrophenol at concentrations of 0·1–0·3mm: (a) had little effect on glucose utilization; (b) depressed synthesis of fatty acids and greatly increased that of lactate; (c) increased the T/14C ratio in fatty acids synthesized from [U-14C,3-T]glucose and decreased that in fatty acids synthesized from [U-14C,4-T]glucose; (d) abolished randomization of 14C from [6-14C]glucose in lactate. 2. Dinitrophenol stimulated oxidation of pyruvate and greatly inhibited the oxidation of lactate. It inhibited lipogenesis from pyruvate and lactate. 3. From the isotope data it was calculated that: (a) dinitrophenol stimulates oxidation via the tricarboxylic acid cycle three- to six-fold; (b) dinitrophenol depresses markedly the operation of the pentose cycle; (c) in the presence of dinitrophenol, NADPH formed in the pentose cycle provides all the hydrogen equivalents for fatty acid reduction, whereas, in its absence, NADPH provides 50–70% of the hydrogen equivalents; (d) in the presence of dinitrophenol, there is an excess of ATP produced in the cytoplasm, which flows into the mitochondria. A reverse flow operates in the absence of dinitrophenol. 4. A balance of formation and utilization of reduced nicotinamide nucleotides in the cytoplasm was established. With dinitrophenol there is some excess of NADH. There are indications that this excess may be transferred into mitochondria in the form of malate. 5. Our results are interpreted to indicate the absence from adipose tissue of the α-glycerophosphate shuttle for transferring reducing equivalents from the cytoplasm to mitochondria. 6. The effects of dinitrophenol are accounted for in terms of decreased ATP concentrations in the cells, leading to marked decrease in pyruvate carboxylation in the mitochondria and depression of fatty acid synthesis in the cytoplasm.
Full text
PDF


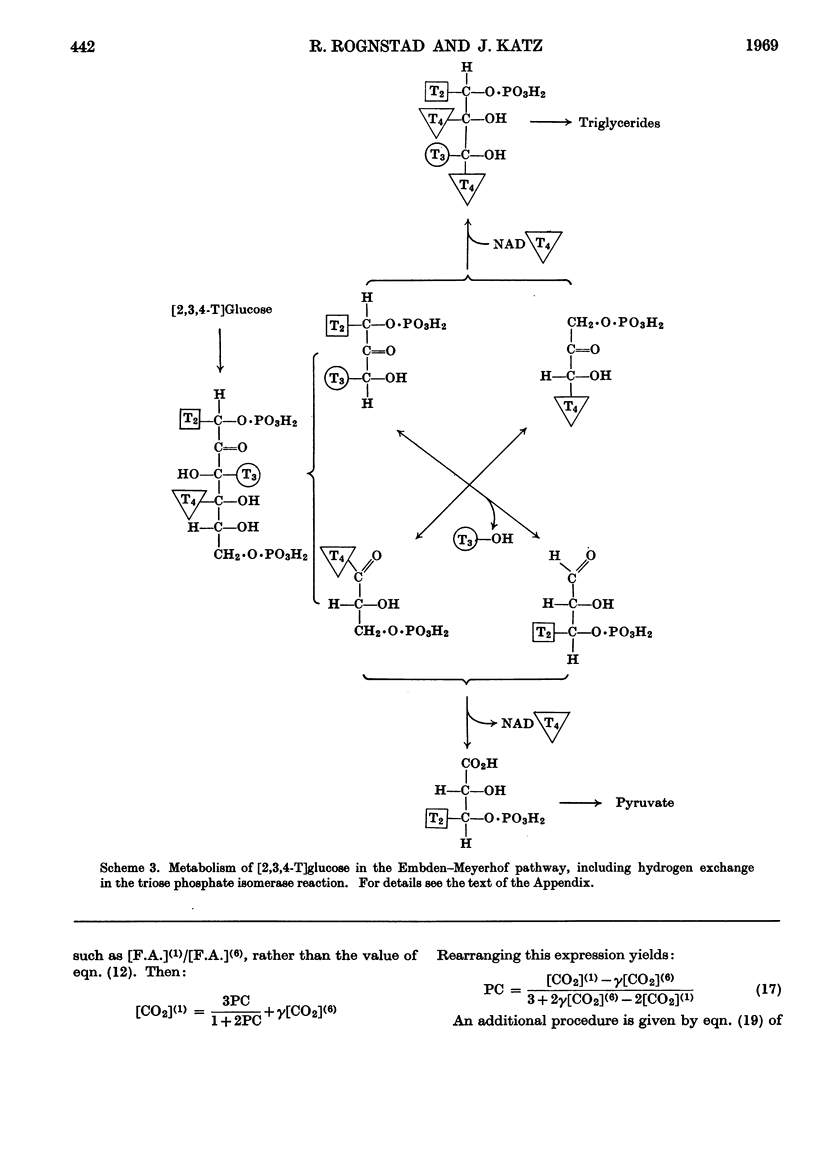
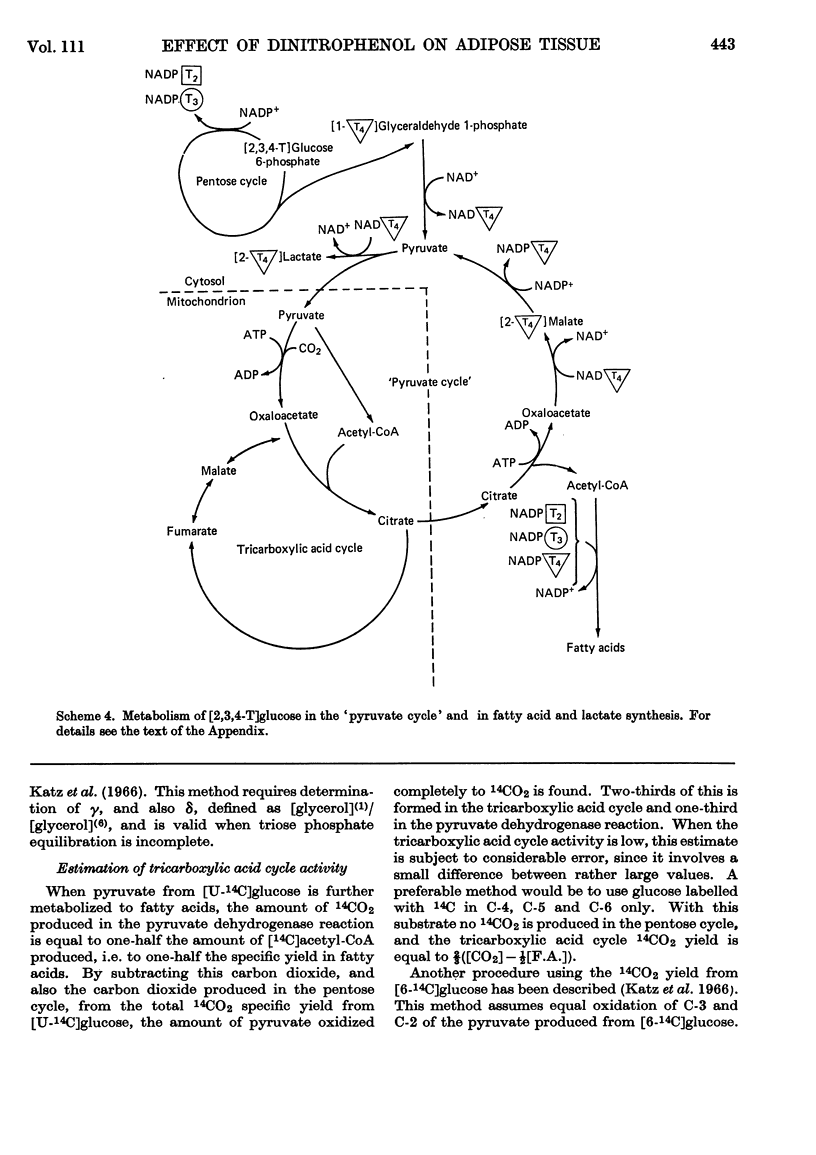

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM B. ENANTIOMORPHIC MONOTRITIATED PRIMARY CARBINOLS OF FRUCTOSE 6-PHOSPHATE: THEIR INTRACELLULAR GENERATION AND METABOLISM BY RAT LIVER SLICES. J Biol Chem. 1964 Jul;239:2102–2105. [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- FRITZ I. B. CARNITINE AND ITS ROLE IN FATTY ACID METABOLISM. Adv Lipid Res. 1963;1:285–334. [PubMed] [Google Scholar]
- Gabriel O., Ashwell G. Biological mechanisms involved in the formation of deoxysugars. I. Preparation of thymidine diphosphate glucose labeled specifically in carbon 3. J Biol Chem. 1965 Nov;240(11):4123–4127. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1960 Aug;235:2165–2177. [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- RIEDER S. V., ROSE I. A. The mechanism of the triosephosphate isomerase reaction. J Biol Chem. 1959 May;234(5):1007–1010. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. Intramolecular hydrogen transfer in the phosphoglucose isomerase reaction. J Biol Chem. 1961 Dec;236:3086–3092. [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]


