Abstract
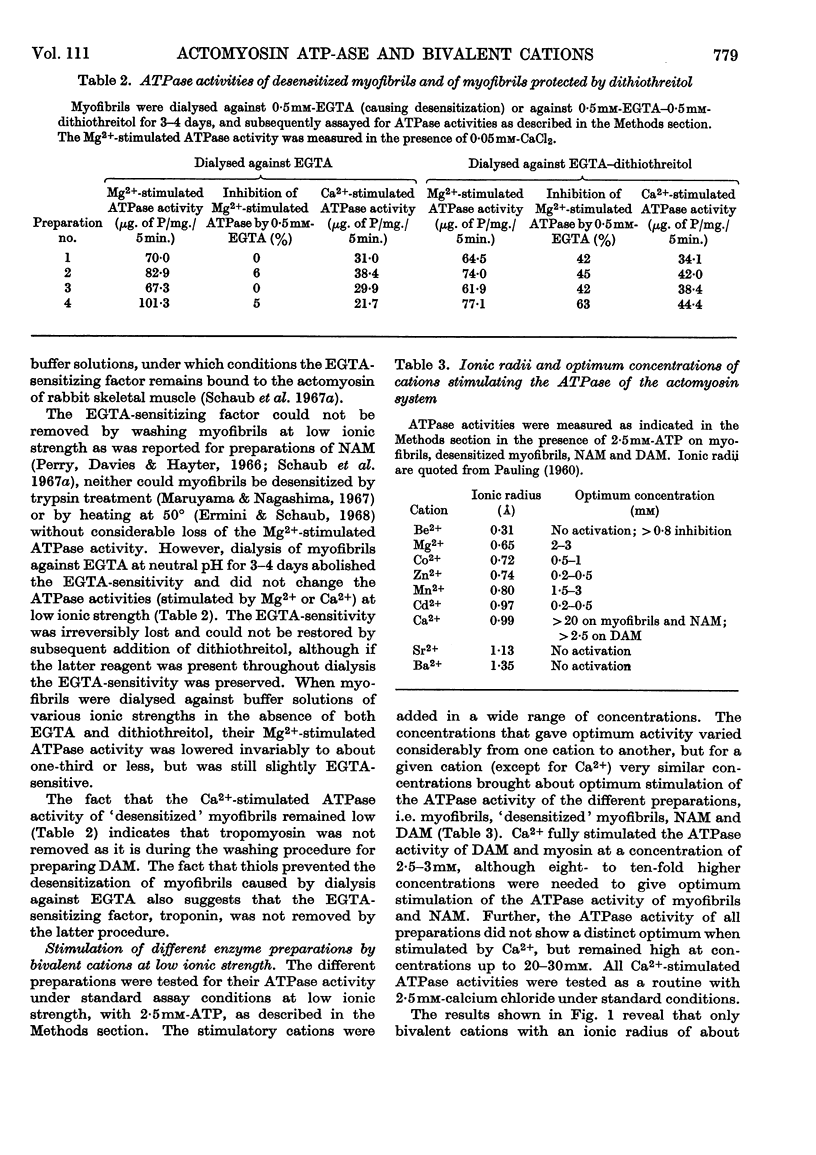
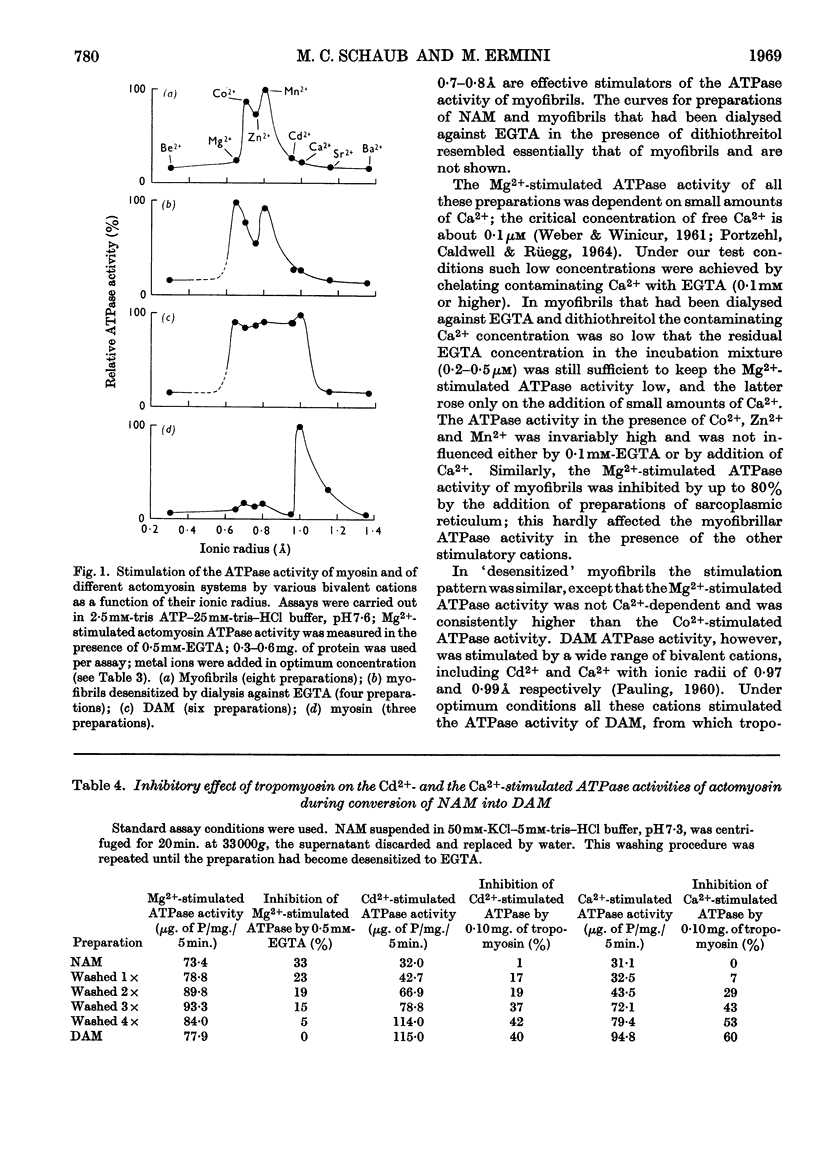
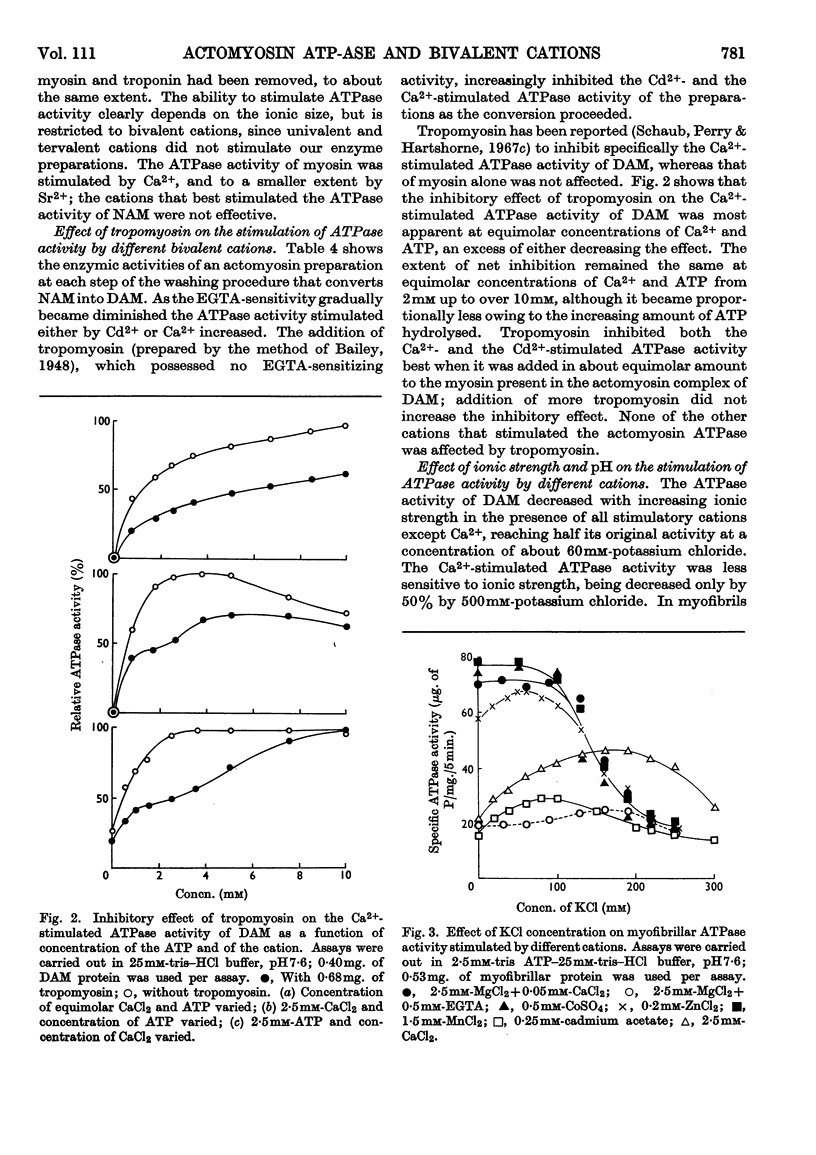
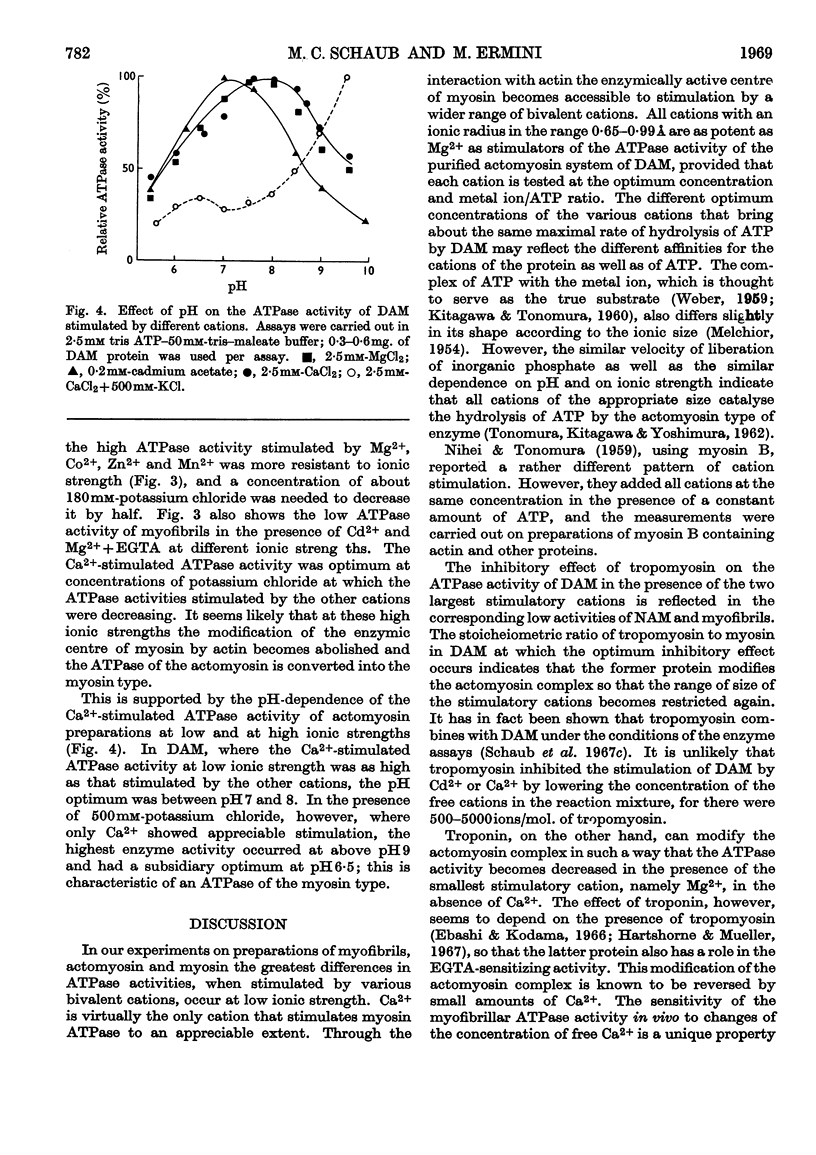
1. After removal of tropomyosin and troponin from the `natural' actomyosin complex, the adenosine triphosphatase activity of the resulting `desensitized' actomyosin is stimulated to the same extent by various bivalent cations with an ionic radius in the range 0·65–0·99å when tested at optimum concentration of the metal ion in the presence of 2·5mm-ATP at low ionic strength and pH7·6. Under identical conditions the adenosine triphosphatase activity of myosin alone is stimulated to an appreciable extent only by Ca2+ (ionic radius 0·99å). 2. Tropomyosin narrows the range of size of the stimulatory cations by inhibiting specifically the adenosine triphosphatase activity of `desensitized' actomyosin when stimulated by Ca2+ or the slightly smaller Cd2+ (ionic radius 0·97å). Tropomyosin has no effect on the adenosine triphosphatase activity of `desensitized' actomyosin when stimulated by the smaller cations, nor on the Ca2+-activated adenosine triphosphatase activity of myosin alone. 3. The adenosine triphosphatase activity of the `natural' actomyosin system (containing tropomyosin and troponin) stimulated by the smallest cation, Mg2+ (ionic radius 0·65å), is low when the system is deprived of Ca2+ but high in the presence of small amounts of Ca2+. This sensitivity to Ca2+ seems to be a unique feature of the Mg2+-stimulated system. 4. The changes in specificity of the myosin adenosine triphosphatase activity in its requirement for bivalent cations caused by interaction with actin, tropomyosin and troponin primarily concern the size of the metal ions. The effects on enzymic properties of myofibrils due to tropomyosin and troponin can be demonstrated at low and at physiological ionic strength.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD G. D., PERRY S. V. The inhibitory action of relaxing-factor preparation on the myofibrillar adenosine triphosphatase. Biochem J. 1960 Nov;77:262–271. doi: 10.1042/bj0770262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZLER E. Binding of calcium and magnesium by the contractile elements. J Gen Physiol. 1955 Jul 20;38(6):735–742. doi: 10.1085/jgp.38.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. P., Avivi Y. Effects of zinc on adenosine triphosphatase activity and superprecipitation of actomyosin from skeletal muscle of rabbit. Arch Biochem Biophys. 1966 Mar;113(3):617–628. doi: 10.1016/0003-9861(66)90239-6. [DOI] [PubMed] [Google Scholar]
- Ermini M., Schaub M. C. Postnatal development of adenosine triphosphatases in red and white rat muscles. Hoppe Seylers Z Physiol Chem. 1968 Oct;349(10):1266–1270. doi: 10.1515/bchm2.1968.349.2.1266. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. Separation and recombination of the ethylene glycol bis (beta-aminoethyl ether)-N,N'-tetraacetic acid-sensitizing factor obtained from a low ionic strength extract of natural actomyosin. J Biol Chem. 1967 Jul 10;242(13):3089–3092. [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MELCHIOR N. C. Sodium and potassium complexes of adenosinetriphosphate: equilibrium studies. J Biol Chem. 1954 Jun;208(2):615–627. [PubMed] [Google Scholar]
- Maruyama K., Nagashima S. Adenosinetriphosphatase activity of trypsin-treated myofibrils and myosin B at low ionic strength. J Biochem. 1967 Sep;62(3):392–395. [PubMed] [Google Scholar]
- Mühlrad A., Kovács M., Hegyi G. The role of Mg2+ in the contraction and adenosine triphosphatase activity of myofibrils. Biochim Biophys Acta. 1965 Oct 18;107(3):567–578. doi: 10.1016/0304-4165(65)90200-x. [DOI] [PubMed] [Google Scholar]
- Nihei T., Morris M., Jacobson A. L. Activation and inhibition of myosin B adenosine triphosphatase by mg++ and ca++ at low concentration of KC1. Arch Biochem Biophys. 1966 Jan;113(1):45–52. doi: 10.1016/0003-9861(66)90154-8. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The chromatography of L-myosin on diethylaminoethylcellulose. Biochem J. 1960 Jan;74:94–101. doi: 10.1042/bj0740094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDEL J. C., GERGELY J. STUDIES ON MYOFIBRILLAR ADENOSINE TRIPHOSPHATASE WITH CALCIUM-FREE ADENOSINE TRIPHOSPHATE. I. THE EFFECT OF ETHYLENEDIAMINETETRAACETATE, CALCIUM, MAGNESIUM, AND ADENOSINE TRIPHOSPHATE. J Biol Chem. 1963 Nov;238:3648–3653. [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. Effect of tropomyosin on the calcium-activated adenosine triphosphatase of actomyosin. Nature. 1967 Aug 5;215(5101):635–636. doi: 10.1038/215635a0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. The adeonosine-triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Jul;104(1):263–269. doi: 10.1042/bj1040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Hartshorne D. J. The effect of tropomyosin on the adenosine triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Dec;105(3):1235–1243. doi: 10.1042/bj1051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONOMURA Y., KITAGAWA S., YOSHIMURA J. The initial phase of myosin A-adenosinetriphosphatase and the possible phosphorylation of myosin A. J Biol Chem. 1962 Dec;237:3660–3666. [PubMed] [Google Scholar]
- WEBER A. On the role of calcium in the activity of adenosine 5'-triphosphate hydrolysis by actomyosin. J Biol Chem. 1959 Oct;234:2764–2769. [PubMed] [Google Scholar]
- WEBER A., WINICUR S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961 Dec;236:3198–3202. [PubMed] [Google Scholar]


