Abstract
Purpose
To assess the efficacy and safety of non-chemotherapy anticancer drugs (immunotherapy or targeted therapy) compared to best supportive care (BSC) or placebo for the treatment of advanced gastric cancer (GC).
Methods
Systematic review of randomized controlled trials (RCTs) searching (May 2022) MEDLINE, EMBASE, CENTRAL, Epistemonikos, ClinicalTrials.gov, and PROSPERO. Certainty of evidence was evaluated following GRADE.
Results
Six RCTs included. Targeted therapies likely result in a slight increase in overall survival (OS) (HR 0.84, 95% CI 0.75, 0.93; moderate certainty) and progression-free survival (PFS) (HR 0.52, 95% CI 0.43, 0.62; moderate certainty). Toxicity had a slightly increased risk (RR 1.19, 95% CI 0.95, 1.48; low certainty). Immunotherapy also showed a likely improvement in PFS (HR 0.60, 95% CI 0.49, 0.73; moderate certainty), while toxicity showed a likely higher risk (RR 2.72, 95% CI 1.24, 5.94; moderate certainty). However, benefits in survival translated to time gains of slightly over a month for OS and less than a month for PFS. No data were reported on performance status (PS), hospital admissions, or quality of life (QoL).
Conclusions
Our study suggests some survival benefits with low toxicity from these treatments, but gains are marginal. Uncertainties persist regarding their impact on QoL and outcomes for patients with poor PS. Caution is advised in treatment selection for advanced GC patients, who should actively participate in decision-making. Future research should include diverse patient populations and assess patient-centered outcomes with consistent comparator groups for BSC.
Trial Registration
The study protocol was registered in OSF (https://doi.org/10.17605/OSF.IO/7CHX6) on 2022–04-01.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12029-024-01155-y.
Keywords: Advanced gastric cancer, Immunotherapy, Targeted therapy, Systematic review, Meta-analysis, Non-chemotherapy anticancer drugs
Introduction
Gastric cancer (GC) remains a significant cause of morbidity and mortality [1], ranking as the fifth most diagnosed and seventh most prevalent cancer worldwide [2, 3]. Over two-thirds of GC is diagnosed at an advanced stage. With an estimated survival of 5% [4], it is a leading cause of cancer death [5, 6].
The emergence of non-chemotherapy anticancer drugs, such as immunotherapy and molecular targeted therapies (IO + targeted), theoretically offers more potential benefits for advanced GC. These benefits might encompass controlling cancer-related symptoms, enhancing survival [4, 7–11], and personalized treatments. [12–14]. However, uncertainties persist regarding patient-centered outcomes beyond survival because achieving enduring disease control remains elusive [15, 16], with anticipated limited overall gains on a mid- or short-term basis [16]. Moreover, these therapies may entail toxicities and adverse events (AEs) that substantially impact patients’ quality of life (QoL) and functional status [17, 18]. Furthermore, the cost-effectiveness of these treatments poses challenges from both clinical and public health perspectives.
On the other hand, because of the uncertainty in the balance of disease control and therapy effects, often best supportive care (BSC) for advanced GC patients is used where there is a focus on symptom management without the use of anticancer drugs. BSC can provide a more comprehensive, early, personalized, and patient-centered approach, focusing on symptom management and improving the QoL [19], involving a less aggressive approach that can be particularly appropriate for advanced GC patients [19, 20].
Despite the increasing use of non-chemotherapy anticancer drugs, a comprehensive review comparing their efficacy and safety to BSC in advanced GC is currently lacking [21]. Thus, this review aims to fill this gap and provide valuable insights for optimal treatment decisions. The objective is to assess the efficacy and safety of non-chemotherapy anticancer drugs—IO + targeted—compared to BSC or placebo for the treatment of advanced gastric cancer.
Methods
Study Design
We conducted a systematic review (SR) in accordance with the Cochrane Handbook for Systematic Reviews and the Preferred Reporting Items for Systematic Reviews and Meta-Analyse (PRISMA) 2020 statement [22, 23] (Appendix 1). This SR represents the third stage of a larger project called the ASTAC-study, which aims to comprehensively synthesize evidence on the effects of systemic anticancer drugs compared to supportive care for individuals with advanced non-intestinal digestive cancers [21, 24–32]. The review protocol was prospectively registered in Open Science Framework (OSF) on April 1, 2022 [33].
Eligibility Criteria
We included only randomized controlled trials (RCTs) that met all the following inclusion criteria: (1) included adult patients diagnosed with advanced or metastatic GC [34], considering studies that included patients with tumors located in various areas such as the cardia, fundus (corpus), body, antrum, pylorus, or gastroesophageal junction (GEJ), and encompassed any histological type, including adenocarcinoma and squamous cell carcinoma; (2) enrolled patients who received immunotherapy or molecular targeted therapy, either as monotherapy or in combination with other treatments, with or without supportive care; and (3) included a comparison group receiving any form of supportive treatment and/or placebo aimed at symptomatic or palliative control without any systemic anticancer treatment [19, 20].
We excluded studies focusing on neuroendocrine, stromal (GIST), or lymphatic neoplasms. Appendix 2 provides a summary of the eligibility criteria.
Search Strategy
The search strategy derived from the broader ASTAC-study [33]. The following four databases were searched: MEDLINE (accessed through PubMed), EMBASE (accessed through OVID), the Cochrane Central Register of Controlled Trials (CENTRAL), and Epistemonikos. The initial search for the ASTAC study was conducted from the inception of each database up to December 2019. In addition, we searched Clinicaltrials.gov and PROSPERO for ongoing or unpublished data. An update of the search was performed in MEDLINE (PubMed) and CENTRAL up until May 2022. Furthermore, we manually reviewed the reference lists of relevant studies to identify any additional relevant articles [35]. Appendix 3 provides the search strategy.
Selection and Data Extraction
Two reviewers performed an independent title and abstract screening. A third author resolved discrepancies when required. Afterward, two reviewers conducted the full-text screening, with a third author solving any disagreement. For all this process, we used the Rayyan platform [36].
Data extraction was carried out independently by two reviewers using a pre-designed and piloted structured template specifically created for this review. Any disagreements during extraction were resolved through discussion, involving a third author if necessary. The outcomes of interest in this SR were as follows: (a) Efficacy: overall survival (OS), progression-free survival (PFS); (b) Safety: toxicity and hospital admissions; and (c) Patient-centered outcomes: symptoms related to the disease, QoL, and quality of end-of-life care.
Quality Assessment
Two reviewers independently assessed the risk of bias (RoB) of included studies using the Cochrane risk-of-bias tool for randomized trials [37]. The following six domains were assessed: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, and 6) selective reporting. The bias score was assessed as a judgment (high, low, or unclear) for individual elements from each domain, and we classified studies as low risk if they showed minimal risk of bias across all key domains, unclear if there were uncertainties in one or more domains, and high risk if one or more domains exhibited high risk of bias. Any disagreements were solved through consensus or, if necessary, with the involvement of a third reviewer.
Statistical Analysis
For the analysis of time-to-event data, specifically OS and PFS, we utilized hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Regarding dichotomous data, such as OS and PFS at 6, 12, and 18 months and toxicity outcomes, we employed risk ratios (RRs) with 95% CIs. For the analysis of continuous data such as OS and PFS, we intended to use either the mean difference (MD) or the standardized mean difference (SMD) with 95% CIs. The MD compares the average difference in outcome values between two treatment groups. To ensure transparency and provide a comprehensive overview, we planned to report the absolute medians for individual studies when available. In cases where multiple studies were included, we presented the range of absolute medians to facilitate comparisons across studies. For the meta-analysis, we utilized a random-effects model. Subgroup analyses were conducted by the treatment group (molecular targeted therapy or immunotherapy). We performed all statistical analysis using Review Manager 5.4.1 software [38].
We expected heterogeneity in the studies due to diverse clinical and methodological factors, including participant characteristics (age, ethnicity, baseline ECOG performance status-PS, and treatment line). Differences in the type of drug interventions (molecular-targeted therapy, immunotherapy, intensity, or dose), outcome measures, and variability in the comparator and follow-up duration could also contribute to the observed heterogeneity. We used forest plots and the I2 index to assess this heterogeneity among the included studies in each analysis. A I2 value exceeding 75% was considered indicative of substantial heterogeneity.
Certainty of the Evidence
We assessed the certainty of evidence of outcomes using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [39]. The results of this assessment were presented in “summary of findings” (SoF) tables. Since our eligibility criteria considered only RCTs, we considered the initial certainty of evidence for each outcome as “high.” Then, we considered the following domains for potentially rating down this certainty: risk of bias, indirectness of the evidence, inconsistency among study results, imprecision of effect estimates, and potential publication bias. Finally, we classified the certainty of the evidence for each outcome as “high”, “moderate”, “low” or “very low” taking into account the downgrading factors mentioned above.
Results
Study Selection
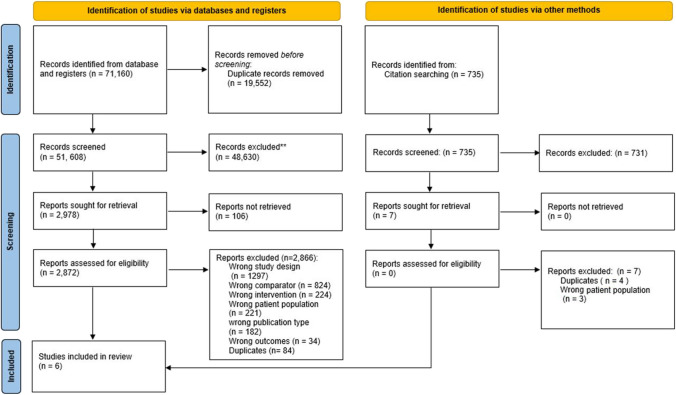
Our search identified 71,160 records for the initial stages of the comprehensive evidence synthesis project ASTAC-study [33]. After removing duplicates, we assessed 51,608 references by title and abstract, excluding 48,630 references. Therefore, we sought 2978 articles for full-text assessment, of which we included six RCTs [8, 12, 14, 40–42]. Figure 1 presents the PRISMA flow diagram, providing a detailed overview of the search results and the screening process.
Fig. 1.
PRISMA 2020 flowchart
Study Characteristics
Table 1 summarizes the general characteristics of the six included double-blind placebo-controlled RCTs. The selected studies spanned a period of 7 years, from 2013 to 2019, with five of them classified as phase III trials, and one as phase II trial [41]. Four trials were conducted in international settings involving multiple countries, while two focused specifically on East Asia [8, 14]. All studies were multicenter, with an average of 83 included centers and 2821 patients.
Table 1.
Characteristics included studies (n = 6)
| Study ID | Fuchs (2014) | Kang (2017) | Kang (2019) | Li (2016) | Ohtsu (2013) | Pavlakis (2016) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial name/ registration | REGARD/NCT00917384 | ONO-4538–12, ATTRACTION-2/NCT02267343 | ANGEL/NCT03042611 | NA/NCT01512745 | GRANITE-1/NCT00879333 | INTEGRATE/ACTRN12612000239864 | ||||||
| Study design | Double-blind placebo control | Double-blind placebo control | Double-blind placebo control | Double-blind placebo control | Double-blind | Double-blind placebo control | ||||||
| Phase | III | III | III | III | III | II | ||||||
| Arms | TT | Control | Immunotherapy | Control | TT | Control | TT | Control | TT | Control | TT | Control |
| Country (ies) | Multinational (29) | Japan, South Korea, and Taiwan | Multinational (13) | China | Multinational (22) | Multinational (4) | ||||||
| Centers (n) | 119 | 49 | 95 | 32 | 137 | 63 | ||||||
| N allocation | 355 | 493 | 460 | 273 | 656 | 147 | ||||||
| 238 | 117 | 330 | 163 | 308 | 152 | 181 | 92 | 439 | 217 | 97 | 50 | |
| Age mean (range) | 60 (52–67) | 60 (51–71) | 62 (54–69) | 61 (53–68) | 60.03 | 59.96 | 58 (23–71) | 58 (28–70) | 62 (20–86) | 62 (26–88) | 60 (32–85) | |
| Female % | 29.0 | 32.0 | 29.4 | 23.3 | 25.0 | 24.2 | 27.0 | 26.0 | 20.0 | 20.0 | ||
| Race/ethnicity | White 76%; Asian 16%; Black 2%; other 6% | White 78%; Asian 15%; Black 2%; other 6% | NR | Asian 67.8%; White 31.7%; Hispanic or Latino 1.1%; unknown or NR: 0.4% | NR | Asian 57%; White 38%; Black < 1%; other 4% | Asian 58%; White 35%; Black < 1%; other 7% | NR | ||||
| ECOG ≥ 2 (%) | 1.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | ||||||
| Follow-up (months) | 12 | 18 | 36 | 30 | 18 | 6 | ||||||
| Line of therapy | 2nd | 3rd or more | 3rd or more | 3rd or more | 2nd and 3rd | 2nd or more | ||||||
| Population |
Adequate hematologic and organ function* Life expectancy of ≥ 12 weeks (GC, including GEJ) |
Life expectancy of ≥ 12 weeks PD-L1 tumor expression was not required for patient enrolment (GC, including GEJ) |
Ability to swallow oral medication Life expectancy of ≥ 12 weeks (GC, including GEJ) |
Adequate hematologic and organ function (GC, including GEJ) |
Adequate hematologic and organ function (GC, including GEJ) |
Adequate hematologic and organ function Ability to swallow oral medication (GC, including GEJ) |
||||||
| Intervention and comparator | Ramucirumab + BSC | Placebo + BSC | Nivolumab | Placebo | Rivoceranib + BSC | Placebo + BSC | Apatinib | Placebo | Everolimus + BSC | Placebo + BSC | Regorafenib + BSC | Placebo + BSC |
| 8 mg/kg IV every 2 weeks | 3 mg/kg IV every 2 weeks. Three infusions one treatment cycle | 700 mg VO QD. Each cycle duration 28 days | 850 mg VO QD. Each cycle duration 28 days | 10 mg VO QD | 160 mg VO (four 40-mg tablets) QD. Each cycle duration 28 days | |||||||
| Outcomes | OS, PFS, FS, toxicity, symptoms related to the disease, QoL | OS, PFS, toxicity, | OS, PFS, toxicity, QoL | OS, PFS, toxicity, QoL | OS, PFS, FS, toxicity, QoL | OS, PFS, toxicity, symptoms related to the disease, QoL | ||||||
| Funding | Private (ImClone Systems) | Private (Ono Pharmaceutical and Bristol-Myers Squibb) | Private (LSK BioPharma) | Private (Merck, Amgen, Hai Wang, Jiangsu Hengrui Medicine, AstraZeneca) | Private (Novartis, Novartis, Sanofi-Aventis) | Public/Private (Bayer HealthCare Pharmaceuticals, Novartis, Genentech, Bayer Schering Pharma, Amgen, Celgene, Pfizer) | ||||||
| Conflict of interest | Declared | Declared | Declared | Declared | Declared | Declared | ||||||
GC gastric cancer, GEJ gastroesophageal junction, NR not reported, ECOG Eastern Cooperative Oncology Group, BSC best supportive care, IV intravenous, VO oral administration, QD once daily, OS overall survival, PFS progression-free survival, FS symptom-related to the disease, QoL quality of life, TT targeted therapies
*Disease progression within 4 months of the last dose of first-line platinum-containing or fluoropyrimidine-containing chemotherapy for metastatic disease, or within 6 months of the last dose of platinum-containing or fluoropyrimidine-containing adjuvant treatment
In terms of participants’ characteristics, the reported median age ranged from 58 to 61 years, covering an age range from 20 to 88 years. Among studies that provided information on participants’ sex, a higher proportion of males was observed, ranging from 68.0 to 80.8%, consistent with known global incidence by sex of approximately 2:1 [43]. Nearly all patients in the trials were fit (ECOG 0–1), with only two studies enrolling a small percentage (1%) of patients with ECOG 2 [12, 40].
All trial participants were pre-treated with at least two lines of therapy. Four of the six studies allowed more than three lines. Notably, none of the studies selected participants based on specific biomarker expressions or programmed death-ligand 1 (PD-L1) scores.
Regarding the evaluated anticancer drugs, five of the RCTs studied molecular targeted therapies, including ramucirumab [12], rivoceranib [42], apatinib [14], everolimus [40], and regorafenib [41]. These targeted therapies were assessed in the context of second-line [12, 40, 41] and third-line [8, 14, 40, 42] therapies. Additionally, one double-blind placebo-controlled RCT tested a third-line immunotherapy regimen involving nivolumab [8]. It is worth mentioning that in the studies conducted by Kang and Li et al., the control group was treated only with placebo [8, 14]. However, in the remaining studies, the control group received a combination of placebo and BSC [12, 40–42].
Ultimately, five of the six studies [8, 12, 14, 40, 42] were fully funded by private entities, with the exception of one [41], which reported both public and private funding.
Risk of Bias
Among the included studies, two were found to have an overall low RoB [12, 14]. Three studies were considered to have an overall unclear RoB [8, 40, 42], and one study was classified as having a high RoB primarily due to incomplete outcome data [41]. Appendix 4 details the RoB assessment.
Outcomes
Overall Survival
The OS analysis included six studies [8, 12, 14, 33–35] (n = 2378). Non-chemotherapy anticancer drugs provide a statistically significant improvement in OS (HR 0.78; 95% CI 0.67, 0.89), both with molecular targeted therapy (HR 0.84, 95% CI 0.75, 0.93; moderate certainty) and immunotherapy (HR 0.62, 95% CI 0.51, 0.75; moderate certainty). Figure 2 presents the forest plot for the meta-analysis of OS. Appendix 8 provides the SoF table.
Fig. 2.
Overall survival in studies comparing non-chemotherapy anticancer drugs to supportive care or placebo for advanced gastric cancer by treatment type
Our analysis of the absolute benefits likely increases the overall OS (MD 1.06; 96% CI 0.61, 1.51), with an MD of 1.04 months (95% CI 0.53, 1.55) for molecular targeted therapy [8, 12, 14, 41, 42] and an MD of 1.12 months (95% CI 0.15, 2.09) for immunotherapy [8]. Appendix 5 shows the meta-analysis for this outcome.
Progression-free Survival
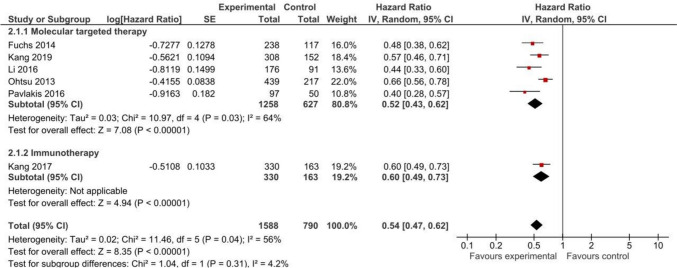
PFS data from all the included RCTs [8, 12, 14, 40–42] were analyzed (n = 2378). Our meta-analysis of five RCTs involving 1885 patients for molecular targeted therapy likely shows an increase in PFS, with an HR of 0.52 (95% CI 0.43, 0.62; moderate certainty). Similarly, immunotherapy probably improves PFS with an HR of 0.60 (95% CI 0.49, 0.73; moderate certainty) based on data from one trial involving 493 patients. The results are visually presented in Fig. 3 and Appendix 8.
Fig. 3.
Progression-free survival in studies comparing non-chemotherapy anticancer drugs to supportive care or placebo for advanced gastric cancer by treatment type
In absolute terms, molecular targeted therapy results in a slight increase in PFS (MD of 0.86 months 95% CI 0.63, 1.35), and immunotherapy probably results in little to no difference (MD of 0.16 months 95% CI 0.05, 0.27). PFS time probably favored the use of non-chemotherapy anticancer drugs, with a (MD of 0.65 months, 95% CI 0.30, 1.00; moderate certainty). Detailed results are shown in Appendix 6.
Toxicity
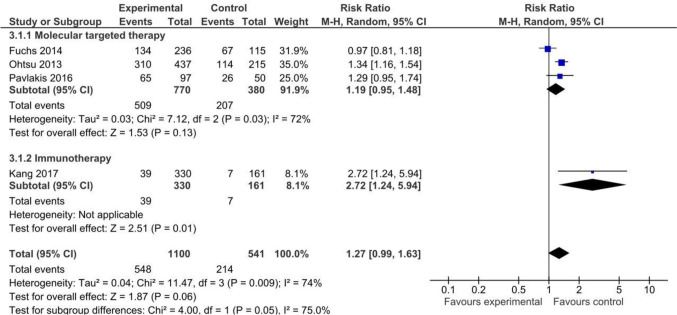
Four [8, 12, 40, 41] of the included RTCs provided data on AEs of grade 3 or higher (n = 1641). Immunotherapy showed a likely higher risk of AEs than placebo with/without BSC, with only one [8] RCT with 46 events (RR 2.72, 95% CI 1.24, 5.94; moderate certainty). On the other hand, molecular targeted therapies pooled analysis of a non-statistically significant increase in the risk of AEs. The analysis based on three [12, 40, 41] trials with 762 events yielded no statistically significant differences (RR 1.19, 95% CI 0.95, 1.48; low certainty). Detailed results are shown in Fig. 4 and Appendix 8.
Fig. 4.
Toxicity in studies comparing non-chemotherapy anticancer drugs to supportive care or placebo for advanced gastric cancer by treatment type
Quality of Life
The assessment of QoL using validated scales (EORTC QLQ-C30 (global and subscales), EORTC QLQ-STO22, EORTC QLQ-OG25, and EQ-5D-3L) was exclusively conducted in the RCTs focusing on molecular targeted therapies [12, 14, 40–42]. Due to heterogeneity in the reported outcomes among the included trials, we describe this outcome narratively. In Fuchs et al. [12] and Ohtsu et al. [40], the addition of molecular targeted therapy (ramucirumab and everolimus, respectively) to BSC compared to placebo plus BSC showed a trend towards a slightly longer time to deterioration in global QoL. However, these differences were not statistically significant. Neither Li et al. [14] nor Pavlakis et al. [41] demonstrated favorable results for QoL. Detailed results are shown in Appendix 7. None of the included studies provided data on hospital admissions, symptoms related to the disease (assessed separately from QoL), or quality of end-of-life care.
Discussion
Main Findings
This SR summarizes the results of six RCTs that assessed the efficacy and safety of non-chemotherapy anticancer drugs, including molecular targeted therapies (apatinib, everolimus, ramucirumab, regorafenib, and rivoceranib) and immunotherapy (nivolumab) with/without BSC compared with placebo with/without BSC in the treatment of advanced GC. The results likely suggest a small improvement in both OS and PFS in relative terms. Nonetheless, given the moderate certainty of evidence, it is crucial to approach these findings thoughtfully. Primarily, the benefits in OS in absolute terms likely result in mean time gains of only around one month (1.04 months for molecular targeted therapy and 1.12 months for immunotherapy), with less than a month of improvement in PFS. Nevertheless, it is worth remarking that a gain within a month or less represents a questionable clinical benefit.
Secondly, all the RCTs focused on advanced GC included only 1% of individuals within the ECOG PS ≥ 2 population. This cautionary approach advises against the inclusion of patients with higher ECOG grades, which, in turn, results in the absence of evidence for this population, which is expected to be the most predominant among advanced CG patients. The lack of data for patients with higher ECOG grades highlights a critical gap in understanding and addressing the needs of these individuals [44, 45]. This emphasizes the necessity for careful consideration of these therapies when offered to patients with poor PS in clinical practice due to the associated probable risks.
Furthermore, BSC was poorly defined in the control group and likely varied substantially across study sites in these large, mostly international trials. Overall, 66% included placebo plus BSC in their control group. This underscores the ongoing issue of lacking standardization in clinical trials, which may lead to overestimating the effect of comparator arms and call into question the validity of the data conclusions [19, 46, 47]. It also raises the possibility that standardizing BSC could further reduce the perceived effectiveness of active treatments. Despite ongoing discussions among scholars and healthcare decision-makers, there is still no universal consensus on defining and delivering BSC in RCTs [19].
Thirdly, despite an increased risk of AEs of grade 3 or higher with non-chemotherapy anticancer drugs (RR of 1.27), no difference in toxicity was found. However, the presence of moderate to substantial heterogeneity suggests that variability among the studies could impact confidence in the estimation and interpretation of results, highlighting the ongoing need to standardize the reporting of AEs. Additionally, we observed no effect of non-chemotherapy anticancer drugs on QoL. However, we were unable to combine study results due to heterogeneity in the measures used. Notably, none of the included studies reported data on other important patient-related outcomes such as hospital admissions, symptoms, or the quality of end-of-life care.
Our Research in Context
In the context of research evaluating the effectiveness of emerging anticancer drugs, numerous studies, including those conducted by our ASTAC-study research group, have been undertaken [48–51]. Our ASTAC group’s investigations [21] have significantly contributed robust evidence, addressing previously unanswered questions and consistently producing reliable outcomes. However, it is important to note that none of these reviews have exclusively focused on evaluating the efficacy of immunotherapy and target therapy primarily in conjunction with supportive care measures. Moreover, this approach underscores patient-centered outcomes beyond mere survival or toxicity, which is particularly pertinent for individuals facing a high risk of short- or medium-term death, which excludes consideration of other anticancer treatments. Furthermore, our results claim for patient-centered outcomes beyond survival or toxicity, which holds particular importance for individuals facing a high risk of short- or medium-term mortality.
Our findings align with similar meta-analyses conducted by Chang et al. in 2017 and Rizzo et al. in 2020 [50, 51]. However, our study stands out by not only encompassing a meta-analysis of immunotherapy and target therapy but also evaluating the certainty of evidence based on the GRADE criteria [39]. Moreover, we incorporated a new study in the third-line treatment [42] that was not considered in their previous analyses. This additional study highlights the ongoing absence of clearly defined standard-of-care regimens in the third- or later-line setting for advanced GC, especially concerning the use of non-chemotherapy anticancer drugs.
Regarding AEs, our study uniquely focuses on AEs of grade 3 or higher severity. It is crucial to note that the distinct side effects associated with immunotherapy and molecular targeted therapy might contribute to the observed high heterogeneity in this aspect. Findings in the network meta-analyses by Cheng et al. in 2019 and Park et al. in 2021 [50, 51] suggest that immunotherapy may be a preferable and beneficial option, considering both efficacy and tolerability. Interestingly, these conclusions closely align with the outcomes of our research.
Strengths and Limitations
This SR is helpful for clinicians and presents a robust assessment of the effect of non-chemotherapy anticancer drugs compared to BSC and/or placebo for patients with advanced GC, using a standardized approach. Notably, the review goes beyond the traditional focus on survival and toxicity outcomes, providing a more comprehensive evaluation of the interventions’ impact on patients’ well-being. A comprehensive search strategy was implemented to ensure inclusivity and minimize selection bias, without language or date restrictions. Additionally, two independent reviewers rigorously conducted the selection, data extraction, and analysis processes. The review adhered to GRADE guidelines [39] to assess the certainty of the evidence for each outcome, further enhancing the transparency and credibility of the findings.
Certain limitations should be acknowledged. Many new treatments in GC for relapsed or refractory disease seek accelerated approval through single-arm phase 2 trials and, therefore, could not be considered in this review. Also, the approval status varies across different markets. For example, ramucirumab has broader approval, whereas rivoceranib is limited to specific regions [52, 53].
It is intriguing to observe that many patients, despite receiving multiple lines of treatment in advanced stages, are often described as having good or perfect PS. Also, many patients will have already received immune checkpoint inhibitors in earlier lines of therapy, further clouding the risk/benefit calculation when considering nivolumab versus BSC, which adds to the uncertainty in clinical decision-making. The skewed representation of younger patients with good ECOG and the exclusion of those with specific comorbidities raise concerns about the generalizability of the findings to a broader target population. It is unclear if elderly patients or those with ECOG > 2 could benefit similarly from third-line treatment with non-chemotherapy anticancer drugs, posing questions about potential errors or bias. Moreover, despite the innovative therapies, there is a scarcity of reports on outcomes beyond survival [54]. Notably, the QoL lacks standardization, making it regrettable that these metrics were not consistently used when comparing novel therapies to BSC focused on improving cancer-related symptoms and QoL.
Furthermore, the substantial heterogeneity in included populations, considering factors such as geographic region, ethnicity, age, sex, and the line of treatment, demands cautious interpretation. Variations in histological patterns and molecular profiles have not been adequately considered in clinical trials [55–57], potentially introducing bias. Additionally, the absence of participant selection based on biomarker expressions limits tailoring treatments to specific subgroups, impacting response variations. In addition to the aforementioned limitations, we highlight the presence of industry-funded studies, which could raise the limitation with the possibility of financial bias [58–60].
Conclusions
Our SR faces challenges in definitively establishing the efficacy and safety of immunotherapy and target therapy anticancer drugs compared to BSC in patients with advanced GC with very good PS. While these drugs likely result in a marginal to modest benefit in OS, PFS compared to BSC and/or placebo, evidence regarding QoL and toxicity remains inconclusive. It is important to interpret these results cautiously and encourage better shared decision-making. Considering the little existing effectiveness, even among the most selected advanced CG patients, it seems reasonable to be quite conservative when offering active treatment options to the majority of CG patients. We highlight the need to include wider populations and to use standardized scales to get better patient-centered outcomes reports with emerging therapies.
Further research, particularly improved RCTs involving diverse populations and better standardization of QoL metrics, symptom control definitions, AEs reporting, and cost evaluation, is essential for gaining a comprehensive understanding of the impact of non-chemotherapy anticancer drugs on patient QoL. This approach is crucial for informed decision-making and optimizing patient care. By considering a broad range of outcomes, decision-making among patients, researchers, and healthcare providers can be enhanced, allowing for personalized treatments tailored to meet the individual needs and values of patients with advanced GC [61].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their gratitude to Pamela Meinardi (Instituto Nacional de Enfermedades Respiratorias Dr. Emilio Coni, Santa Fe, Argentina), Juan Irassar (Ministerio de Salud de la Provincia de Buenos Aires, La Plata, Argentina) for their collaboration in study selection and data extraction. Also, the authors would like to acknowledge Santiago Jami (Universidad Iberoamericana del Ecuador, Quito, Ecuador) and Iratxe Urreta (Epidemiology Unit, Donostia University Hospital, Donostia, Spain) for their collaboration, particularly their feedback and clinical guidance on methodology, clinical approaches, and data extraction.
Abbreviations
- AEs
Adverse events
- ASTAC
Appropriateness of Systemic Oncological Treatments for Advanced Cancer
- BSC
Best supportive care
- CI
Confidence interval
- ECOG
Eastern Cooperative Oncology Group
- GC
Gastric cancer
- GEJ
Gastroesophageal junction
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- HR
Hazard ratio
- IO
Immunotherapy
- OS
Overall survival
- PFS
Progression-free survival
- PS
Performance status
- QoL
Quality of life
- RCTs
Randomized clinical trials
- RoB
Risk of bias
- RR
Relative risk
- SoF
Summary of findings
- SR
Systematic review
Author Contribution
AM, MS, JB, and XB conceived and designed research. AM, MS, JB, OS, AS, MI, CP and LL conducted search and extracted the data. OS and LL conducted article quality assessment. All authors wrote the manuscript. All authors read and approved the manuscript.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. This study has been funded by Instituto de Salud Carlos III through the project “PI18/00034” (co-funded by European Regional Development Fund “A way to make Europe”). Marilina Santero is funded by Instituto de Salud Carlos III through the contract “FI19/00335” (co-funded by European Social Fund “Investing in your future”).
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval and Consent to Participate
This study is based on secondary data and does not involve primary data collection or human participants. So, ethics approval and consent to participate are not applicable.
Competing Interests
The authors declare no financial or non-financial competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Zhang P-F, Xi H-Q, Wei B, Chen L, Tang Y. Recent advances in the diagnosis, staging, treatment, and prognosis of advanced gastric cancer: a literature review. Front Med. 2021;8:744839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–49. [DOI] [PubMed] [Google Scholar]
- 5.Alshehri A, Alanezi H, Kim BS. Prognosis factors of advanced gastric cancer according to sex and age. World J Clin Cases. 2020;8:1608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer of the stomach - cancer stat facts [Internet]. SEER. Available from: https://seer.cancer.gov/statfacts/html/stomach.html . Accessed 28 Nov 2023.
- 7.Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim T-Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 9.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2018;2019(19):1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Full access. NCCN Guidelines® Updates: Gastric Cancer. J Natl Compr Canc Netw. 2022;20:xiii–xiv.
- 11.Shen J, Wang Z. Recent advances in the progress of immune checkpoint inhibitors in the treatment of advanced gastric cancer: a review. Front Oncol. 2022;12:934249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y-K, Boku N, Kang WK, Yoon HH, Cascinu S, Al-Batran S-E, Houston S, Park CH, McGinn AN, Chau I. A prospective, randomized, double-blinded, placebo-controlled, phase III study to evaluate the efficacy and safety of apatinib plus best supportive care (BSC) compared to placebo plus BSC in patients with advanced or metastatic gastric cancer: the ANGEL study. J Clin Orthod. 2017;35:TPS4138. [Google Scholar]
- 14.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebenhüner AR, De Dosso S, Helbling D, Astaras C, Szturz P, Moosmann P, Pederiva S, Winder T, Von Burg P, Borner M. Advanced gastric cancer: current treatment landscape and a future outlook for sequential and personalized guide: Swiss expert statement article. Oncol Res Treat. 2021;44:485–94. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Casarett D, Corcoran A, Desai K, Li Q, Chen J, Langer C, Mao JJ. Utilization of supportive and palliative care services among oncology outpatients at one academic cancer center: determinants of use and barriers to access. J Palliat Med. 2012;15:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): patient version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2020.
- 19.Rubiales ÁS, Sanz Rubiales Á, Sánchez-Gutiérrez ME, Flores Pérez LA, del Valle Rivero ML. How is best supportive care provided in clinical trials for patients with advanced cancer? A review of registered protocols of clinical trials. Curr Oncol. 2020;27:100–5. 10.3747/co.27.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo L, Urbauer DL, Bruera E, Hui D. Recommendations for supportive care and best supportive care in NCCN clinical practice guidelines for treatment of cancer: differences between solid tumor and hematologic malignancy guidelines. Support Care Cancer. 2021;29:7385–92. 10.1007/s00520-021-06245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santero M, Pérez-Bracchiglione J, Acosta-Dighero R, Meade AG, Antequera A, Auladell-Rispau A, Quintana MJ, Requeijo C, Rodríguez-Grijalva G, Salas-Gama K, et al. Efficacy of systemic oncological treatments in patients with advanced esophageal or gastric cancers at high risk of dying in the middle and short term: an overview of systematic reviews. BMC Cancer. 2021;21:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.5 (updated August 2024). Cochrane. 2024. Available from https://www.training.cochrane.org/handbook. Accessed 10 Dec 2024. [DOI] [PMC free article] [PubMed]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol. 2021;74:790–9. [DOI] [PubMed] [Google Scholar]
- 24.Marilina S, Adriana M, Anna S, Roberto A-D, Nicolás M, Jesús QM, Javier B, Carolina R, Josefina S, Gerardo RG, et al. Comparative analysis of systemic oncological treatments and best supportive care for advanced gastresophageal cancer: a comprehensive scoping review and evidence map. J Evid Based Med. 2023;16:216–36. [DOI] [PubMed] [Google Scholar]
- 25.Santero M, Requeijo C, Quintana MJ, Rodríguez D, Bottaro D, Macias I, Pericay C, Farina N, Blanco JM, Urreta-Barallobre I, et al. How appropriate is treating patients diagnosed with advanced esophageal cancer with anticancer drugs? A multicenter retrospective cohort Spanish study. Clin Transl Oncol. 2024. 10.1007/s12094-024-03436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santero M, de Mas J, Rifà B, Clavero I, Rexach I, Bonfill CX. Assessing the methodological strengths and limitations of the Spanish Society of Medical Oncology (SEOM) guidelines: a critical appraisal using AGREE II and AGREE-REX tool. Clin Transl Oncol. 2024;26:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santero M, Meade AG, Acosta-Dighero R, González L, Melendi S, Solà I, Urrútia G, Quintana MJ, Bonfill CX. European clinical practice guidelines on the use of chemotherapy for advanced oesophageal and gastric cancers: a critical review using the AGREE II and the AGREE-REX instruments. Clin Transl Oncol. 2022;24:1588–604. [DOI] [PubMed] [Google Scholar]
- 28.Requeijo C, Bracchiglione J, Meza N, Acosta-Dighero R, Salazar J, Santero M, Meade A-G, Quintana MJ, Rodríguez-Grijalva G, Selva A, et al. Anticancer drugs compared to no anticancer drugs in patients with advanced hepatobiliary cancer: a mapping review and evidence gap map. Clin Epidemiol. 2023;15:1069–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar J, Bracchiglione J, Acosta-Dighero R, Meza N, Meade A-G, Quintana MJ, Requeijo C, Rodríguez-Grijalva G, Santero M, Selva A, et al. Systemic oncological treatments in patients with advanced pancreatic cancer: a scoping review and evidence map. Support Care Cancer. 2023;31:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bracchiglione J, Rodríguez-Grijalva G, Requeijo C, Santero M, Salazar J, Salas-Gama K, Meade A-G, Antequera A, Auladell-Rispau A, Quintana MJ, et al. Systemic oncological treatments versus supportive care for patients with advanced hepatobiliary cancers: an overview of systematic reviews. Cancers. 2023;15. 10.3390/cancers15030766 [DOI] [PMC free article] [PubMed]
- 31.Salazar J, Pérez-Bracchiglione J, Salas-Gama K, Antequera A, Auladell-Rispau A, Dorantes-Romandía R, Meade AG, Quintana MJ, Requeijo C, Rodríguez-Grijalva G, et al. Efficacy of systemic oncological treatments in patients with advanced pancreatic cancer at high risk of dying in the short or medium-term: overview of systematic reviews [Internet]. European Journal of Cancer. 2021;154:82–91. Available from: 10.1016/j.ejca.2021.05.034 [DOI] [PubMed]
- 32.Salazar J, Bracchiglione J, Savall-Esteve O, Antequera A, Bottaro-Parra D, Gutiérrez-Valencia M, Martínez-Peralta S, Pericay C, Tibau A, Bonfill X, et al. Treatment with anticancer drugs for advanced pancreatic cancer: a systematic review. BMC Cancer. 2023;23:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez JB, Salazar J, Santero M, Requeijo C, Grijalva GR, Acosta-Dighero R, Meza N, Salas-Gama K, Olid AS, Meade A-G, et al. Efficacy of systemic oncological treatments in patients with advanced, non-intestinal digestive cancer at high risk of dying in the middle and short term: evidence synthesis (ASTAC-study) [Internet]. 2022. Available from: https://osf.io/7chx6/. Accessed 10 Dec 2024
- 34.Liu J-Y, Peng C-W, Yang X-J, Huang C-Q, Li Y. The prognosis role of AJCC/UICC 8th edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10:292–303. [PMC free article] [PubMed] [Google Scholar]
- 35.Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In: Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering. New York: Association for Computing Machinery; 2014;1–10.
- 36.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Cochrane Collaboration. RevMan (Review Manager) [Computer software, Version 5.4]. The Cochrane Collaboration. 2020.
- 39.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtsu A, Ajani JA, Bai Y-X, Bang Y-J, Chung H-C, Pan H-M, Sahmoud T, Shen L, Yeh K-H, Chin K, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang Y-K, Bang Y-J, Alcindor T, O’Callaghan CJ, Burnell MJ, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol. 2016;34:2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang Y-K, Kang WK, Di Bartolomeo M, Chau I, Yoon HH, Cascinu S, Ryu M-H, Kim JG, Lee K-W, Oh SC, et al. Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens. Ann Oncol. 2019;30:v877–8. [Google Scholar]
- 43.Lou L, Wang L, Zhang Y, Chen G, Lin L, Jin X, Huang Y, Chen J. Sex difference in incidence of gastric cancer: an international comparative study based on the Global Burden of Disease Study 2017. BMJ Open. 2020;10:e033323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hess LM, Smith D, Cui ZL, Montejano L, Liepa AM, Schelman W, Bowman L. The relationship between Eastern Cooperative Oncology Group performance status and healthcare resource utilization among patients with advanced or metastatic colorectal, lung or gastric cancer. J Drug Assess. 2020;10:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnuson A, Bruinooge SS, Singh H, Wilner KD, Jalal S, Lichtman SM, Kluetz PG, Lyman GH, Klepin HD, Fleury ME, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-Friends of Cancer Research Performance Status Work Group. Clin Cancer Res. 2021;27:2424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nipp RD, Currow DC, Cherny NI, Strasser F, Abernethy AP, Zafar SY. Best supportive care in clinical trials: review of the inconsistency in control arm design. Br J Cancer. 2015;113:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zafar SY, Currow DC, Cherny N, Strasser F, Fowler R, Abernethy AP. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol. 2012;13:e77-82. [DOI] [PubMed] [Google Scholar]
- 48.Chan W-L, Yuen K-K, Siu SW-K, Lam K-O, Kwong DL-W. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:68–81s. [DOI] [PubMed] [Google Scholar]
- 49.Rizzo A, Mollica V, Ricci AD, Maggio I, Massucci M, Rojas Limpe FL, Fabio FD, Ardizzoni A. Third- and later-line treatment in advanced or metastatic gastric cancer: a systematic review and meta-analysis. Future Oncol. 2020;16:4409–18. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Cai M, Shuai X, Gao J, Wang G, Tao K. Systemic therapy for previously treated advanced gastric cancer: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2019;143:27–45. [DOI] [PubMed] [Google Scholar]
- 51.Park S, Nam CM, Kim S-G, Mun JE, Rha SY, Chung HC. Comparative efficacy and tolerability of third-line treatments for advanced gastric cancer: a systematic review with Bayesian network meta-analysis. Eur J Cancer. 2021;144:49–60. [DOI] [PubMed] [Google Scholar]
- 52.Yokota T, Bendell J, LoRusso P, Tsushima T, Desai V, Kenmotsu H, Watanabe J, Ono A, Murugesan B, Silva J, et al. Impact of race on dose selection of molecular-targeted agents in early-phase oncology trials. Br J Cancer. 2018;118:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang A, Yang X-R, Chung W-Y, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macauley, R.C. Ethics in palliative care: a complete guide. Oxford University Press; 2018.
- 55.Kahraman S, Yalcin S. Recent advances in systemic treatments for HER-2 positive advanced gastric cancer. Onco Targets Ther. 2021;14:4149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23:565–78. [DOI] [PubMed] [Google Scholar]
- 57.Pan Y, Si H, Deng G, Chen S, Zhang N, Zhou Q, Wang Z, Dai G. A Composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-PD-1 antibodies. Front Oncol. 2021;11:798415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chopra SS. MSJAMA: Industry funding of clinical trials: benefit or bias? JAMA. 2003;290:113–4. [DOI] [PubMed] [Google Scholar]
- 59.Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: a scoping review. Am J Public Health. 2018;108:e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2:MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinn KL, Krahn M, Stukel TA, Grossman Y, Goldman R, Cram P, Detsky AS, Bell CM. No time to waste: an appraisal of value at the end of life. Value Health. 2022;25:1902–9. 10.1016/j.jval.2022.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.