Abstract
Metals have been used for many centuries, but their nutritional and toxic effects have been investigated since the last century. The common toxic heavy metals (THM) include mercury, lead, chromium cadmium, and arsenic. As human exposure to THM increasingly causes systemic and organ complications, it seems required to review the recent advances of treatment of the toxic metals.
Despite the current knowledge of the hazards of heavy metals, there is still high incidents of their poisonings particularly in developing countries. In this review, after an introduction, we briefly describe the routes of exposure, clinical features and prognosis of each metal poisoning. Then, review the different treatments for each metal with particular attention to recent advances in the treatment of both acute and chronic poisonings. The main antidotes for all THM are still chelating agents, but new agents were developed over the past decades and have been used successfully for the THM poisonings. Dimercaptosuccinic acid (DMSA) known as succimer has been prescribed as a safe oral chelator in lead poisoning. Similarly, dimercapto-propanesulfonic acid (DMPS) has also revealed fewer side effects than the old chelating agents. The two are currently gaining increased acceptance among clinical toxicologists. However, there is no specific antidote for mercury poisoning. Dimercaprol is almost no longer used as an antidote of choice in the treatment of chronic THM poisoning.
Comparison of clinical management of intoxication by the five heavy metals reveals similar treatment strategies. On the other hand, some of them require specific interventions to reduce the toxicity. Because of drawbacks in the application of commonly known chelating agents, treatment with bioactive compounds which have antioxidant and anti-inflammatory properties has been the subject of much interest in recent research. However, despite the promising results observed in experimental animals, clinical trials on their clinical therapeutic benefits have not been yet successful and need further studies to determine their efficacy and safety in humans. Development of less toxic chelating agents are still under investigations. Moreover, the development of orally administrable chelating agents for home health care would likely be of great interest for future research.
Keywords: Heavy metals, Toxic heavy metals, Complications, Poisoning, Treatment, Chelation therapy, Chelating agents, Chelators, Toxic metals, Antidote, Intoxication, Antioxidant
1. Introduction
Heavy metals are widespread toxicants that humans have come in contact with since last centuries. Human exposure to these metals can be arisen due to natural or anthropogenic activities. While their use is beneficial for a facilitated life (Table 1), environmental exposure can be hazardous and cause toxic adverse effects [[1], [2], [3]]. However, some metals that are considered as essential micronutrients are responsible for the important biochemical processes in the body. Copper is involved in the enzymes superoxide dismutase (SOD), cytochrome oxidase, alcohol dehydrogenase (ALD), alkaline phosphatase (ALP), ascorbate oxidase, tyrosinase and lysyl oxidase [[4], [5], [6]]. Copper also plays a crucial role in iron absorption and heme biosynthesis [6]. Cr3+ is a component of the glucose tolerance factor and plays a decisive role in insulin metabolism [7]. Selenium is the prosthetic group of glutathione peroxidase. Other metals like iron, zinc, magnesium, sodium, potassium and calcium are essential for the body [8]. Nevertheless, Cr6+ and the high levels the essential and nutrient metals are toxic and should be monitored as clinically indicated [9]. In this review, we considered five common toxic heavy metals (THM); including Mercury (Hg), lead (Pb), chromium (Cr), cadmium (Cd) and arsenic (As).
Table 1.
Common THM compounds, their chemical formula and applications.
| Chromium (Cr) compounds | Chemical Formula | Uses |
|---|---|---|
| Barium chromate (Cr6+) | BaCrO4 | Anticorrosive, safety matches, paint pigment |
| Calcium chromate (Cr6+) | CaCrO4 | Batteries, metallurgy |
| Chromic acid (Cr6+) | H2CrO4 | Oxidizer, electroplating, |
| Lead chromate (Cr6+) | PbCrO4 | Paints and dye Yellow pigment |
| Potassium dichromate (Cr6+) | K2Cr2O7 | Leather tanning, oxidizer of organic compounds, porcelain painting |
| Chromic chloride (Cr3+) | CrCl3 | Total parenteral nutrition |
| Chromium picolinate (Cr3+) | C18H12CrN3O6 | Nutritional supplement |
| Chromic fluoride (Cr3+) | CrF3 | Protecting against moth for wool and silk, fixing material in dye industry |
| Chromic oxide (Cr3+) | Cr2O3 | Metal plating, wood treatment |
| Chromite ore (Cr3+) | FeCr2O4 | Water tower treatment |
| Cadmium (Cd) compounds | Chemical Formula | Uses |
| Cadmium carbonate | CdCO3 | Paints and dye pigment |
| Cadmium chromate | CdCRO4 | Chemical manufacturing |
| Cadmium sulfide | CdS | Solar cells, light emitting diodes |
| Cadmium oxide | CdO | Paints and dye pigment |
| Cadmium telluride | CdTe | Solar cells |
| Mercury (Hg) compounds | Chemical Formula | Uses |
| Mercury acetate | Hg(CH3CO2)2 | Chemical and pharmaceutical manufacturing |
| Mercury chloride | HgCl2 | Disinfectant, photography |
| Methylmercury chloride | CH3HgCl | Chemical Manufacturing |
| Phenylmercury acetate | CH3CO2HgC6H5 | Used in pesticides |
| Lead (Pb) compounds | Chemical Formula | Uses |
| Lead acetate | Pb(CH3CO2)2 | Chemical manufacturing, pigment production |
| Lead nitrate | Pb(NO3)2 | Manufacture of explosives, pigment production, chemical manufacturing |
| Lead chloride | PbCl2 | Chemical manufacturing, ceramics manufacture |
| Lead thiocyanate | Pb(SCN)2 | Manufacture of explosives |
| Lead oxides (lead tetroxide and lead dioxide) | Pb3O4 and PbO2 | Battery and pigment manufacturing |
| Arsenic (As) compounds | Chemical Formula | Uses |
| Sodium arsenite | NaAsO2 | Used in pesticides |
| Arsine | AsH3 | Chemical warfare agent, metals refining industries |
| Arsenates of sodium, calcium, potassium, lead | AsO4− | Disinfectant, paper industries |
THM begin to accumulate in the body following a close contact in the environment via either chronic or acute exposure. Under these conditions and by the accumulation of THM in the body, the harmful effects of their accumulation appear. Thus, millions of children and adults might be affected around the world [10]. Adverse effects of THM may cause loss of important nutritional minerals, disruption of normal physiological activities due to interference/inhibition in enzyme function, damage to the central nervous system (CNS), hematological complications, occurrence of delayed pain and emerging of cancers [11]. Prevention of THM exposure is the first measure of treatment that must be taken. For those intoxicated by THM, a series of therapeutic measures should be performed to lower the blood/serum level of the toxic elements. These can include combatting dietary deficiencies, applying symptomatic treatment and supportive care, and prescribing appropriate medications as required [10]. Chelators are well known pharmaceutical antidotes for the treatment of THM that are being widely used in this regard. While some chelating agents are used for more than one THM, the use of specific chelator for the treatment of poisoning by some THM is advocated. In this study, we aimed to review the recent advances in treatment of poisoning related to the five important and commonly used THM.
2. Methods
This narrative review gives a comprehensive context and a specific overview of the present research data on recent advances on the clinical management of the five THM which are of great public health significance. The common therapy of these metals with specific focus on the new drug treatment in recent years for moderate to severe THM poisonings was reviewed. The aim of the present study was to discuss and compare the data on treatment protocols from the primary scientific literature databases including Scopus, PubMed, Web of Science (ISI) and Google Scholar. The search terms were included “treatment”,” management”, “therapy”, “chelators”, “chelating agents”, “antidotes”, “mercury”, “lead”, “chromium”, “cadmium”, “arsenic”, “toxicity”, “poisoning” and “intoxication”. The literature was searched for both animal and human studies involving methods for treatment related to the five THM and any related possible therapies. Unpublished data and letter to the editor were excluded. Case reports, conference papers, and books were not included into our study. Only English articles were included. We preferred to search and review the articles published within the last 20 years. Symptomatic treatment, supportive care, antidotal therapy, and chelating therapy for each metal were extracted from the literature. The similarities and differences of these treatments were compared.
Each of the five THM was drafted by one of the authors (numbers 2–6) who had vast experience in clinical management of THM poisoning. Each draft was first reviewed by the first and corresponding authors and revised by the drafters. Introduction, methods, general discussion and abstract were prepared by the first and corresponding authors and draft of the full papers after several revisions were finally reviewed by the all authors. The first and corresponding authors made the final version for submission.
3. THM
3.1. Mercury (Hg)
3.1.1. Size of the problem and preventive measures
There are relatively large amounts of vapors of various mercury compounds in the atmosphere mainly due to human activities. They are always in rotation and exchange with high amounts of mercury in the water and soil of different regions of the earth. Therefore, the global anthropogenic Hg emission was estimated more than 1900 metric tons in 1995 [12,13]. Due to above-mentioned points and the many uses of mercury in today's life and industry (which is newly a little more limited), getting mercury from the environment is inevitable and thus, the absolute prevention of mercury pollution seems impossible. On the other hand, mercury bioaccumulation in humans can cause adverse health problems [14,15]. Therefore, prevention of mercury exposure in any way can be very helpful in this regard. However, our exposure to Hg happens mainly through the consumption of MeHg contaminated fish [13,16,17]. But practical measures can be implemented in these fields which include:
-
I.
Public education (especially for children) can play an important role in preventing mercury contamination [18]. Trying not to pollute the environment (water, soil, air and living environment in general) with mercury should be the main focus of these trainings. In addition, learning the methods of cleaning the environment from mercury contamination should be the main focus of specialized training in related professions. Training of the workers in the industries that Hg is used e.g. fluorescent bolb plants is of great importance. They should use protective devices, particularly mask with special Hg filter. The workers should learn how to avoid metal mercury exposure and how to clean Hg in the work environment in case of contamination. They should learn how to prevent their own contamination and minimize the possibility of contamination of the work environment and other colleagues. The necessary prevention facilities of Hg exposure in the workplace should be performed [19].
-
II.
In recent years to avoid Hg exposure, new alternative technologies were used to replace the old methods. For instance, Hg free digital pressure gauges, and Hg-free agricultural pesticides and fungicides have been applied. Prohibiting of amalgam which contained around 50 % Hg in restorative dentistry and using composite instead is another example. According to the World Health Organization (WHO), the main exposure to elemental mercury is from dental amalgams [14,15,20].
-
III.
The use of inorganic mercury in skin lightning products can have diverse effects. Mercurous (Hg+) and mercuric (Hg2+) salts are common forms of inorganic Hg human exposure. Depending the exposed Hg amount, from no symptoms to severe signs may occur [[21], [22], [23], [24], [25], [26]]. Therefore, producing and encouraging consumers to use Hg-free cosmetics should be considered.
-
IV.
The consumption of food grown in artificial environments such as farmed fish can be very effective in reducing the intake and accumulation of mercury in the body especially when precise scientific methods are used to grow them.
Oxidative stress is an important molecular mechanism of Hg toxicity. Hg may induce oxidative stress through several mechanisms including ROS generation, GSH depletion, alteration of antioxidant enzymes, mitochondrial dysfunction, and inflammatory responses. Vascular endothelial growth factor (VEGF) upregulation in endothelial cells following MeHg exposure can induce endothelial inflammation. Such endothelial/vascular dysfunction can increase the risk of cardiovascular disease [27]. Oxidative stress resulting from inorganic Hg deposits in the brain via the increase of lipoperoxidation, MDA and nitrite concentration, and a decrease of the total antioxidant capacity leads to fine motor dysfunction linked to imbalance of redox status in the cortex, apoptosis, and cellular death [28]. An in vitro treatment of human embryonic kidney epithelial cell line with HgCl2 showed a decrease in cell viability and oxidative stress mediated by the mitochondrial disorder and upregulation mitochondrial fission at the cellular level [29].
3.1.2. Acute mercury poisoning
Oral ingestion of Hg0 rarely causes acute toxic effects, it is absorbed as low as 0.01 % of the dose in the gastrointestinal (GI) tract. Ingestion of a single dose of elemental mercury presents a negligible risk of toxicity [19]. Acute intoxication is commonly linked to the inhalation of Hg0 or ingestion of inorganic mercury. Acute Hg inhalation can lead to pulmonary dysfunction and other organ disorders. Emerging signs and symptoms are fever, shortness of breath, chills, metallic taste and pleuritic chest pain. Stomatitis, confusion, lethargy and vomiting are other possible symptoms. Pulmonary complications of Hg inhalation may progress to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum and interstitial fibrosis. Acute poisoning of inorganic mercury or mercuric salts is usually the cause of oral ingestion. The acute signs and symptoms of toxicity caused by inorganic mercury or mercuric salt are primarily due to their corrosive properties. The acute presentation can lead to various signs, such as ashen-gray mucous membranes, hematochezia (bloody stool), severe abdominal pain, vomiting and hypovolemic shock. Systemic effects usually start several hours after ingestion, including mucosal inflammation, metallic taste, gingival irritation, renal tubular necrosis and loosening of teeth [19,[30], [31], [32], [33]]
3.1.3. Chronic mercury poisoning
Chronic intoxication is more common from organic Hg exposures. Chronic exposure can also result from delayed occupational exposure to Hg0 that can be transformed into the inorganic form in the body. The other long-term exposures include topical application of mercurial salts and the chronic use of diuretics or cathartics containing Hg. Chronic Hg exposure can cause various renal, neurological, psychological and cutaneous disorders. Non-specific symptoms include anorexia, weight loss, fatigue and muscular weakness that could be indicative of multiple diseases [30]. Chronic poisoning markedly affects the central nervous system and the kidneys. Tremors are present in the facial muscles, eyelids and late in the limbs. Irritability, fears, shyness, loss of confidence are commonly observed. Frank nephrotic syndrome can be developed following chronic Hg exposure. The most common indication of renal disorders is proteinuria-reflecting glomerular damage [19,[30], [31], [32], [33]].
3.1.4. Prognosis for mercury poisoning
Like all other toxicities, the prognosis of poisoning depends on various factors. a good prognosis may be achieved by early detection. Besides, the prognosis relies on the duration of exposure, Hg concentration and the severity of clinical manifestations [26]. The prognosis for Hg exposure is highly variable and it depends on the level and duration of exposure. High exposure can cause coma and death. Other minor symptoms may not be diagnosed and resolve over time. Neurologic disorders which can be delayed in the presentation may last for decades without diagnosing of Hg poisoning, unless the physician considered the history of Hg exposure. Fetus and children are highly susceptible to Hg exposure. It may produce severe toxicity leading to death or permanent neurologic deficits such as mental retardation [34]. In nephrotic syndrome following the Hg toxicity, using chelating agents D-penicillamine and 2,3-dimercapto-1-propanesulfonic acid (unithiol, DMPS) alone or in combination with other treatments has been shown to have a good prognosis with improvement of clinical manifestations and lab parameters [26,[35], [36], [37], [38]].
3.1.5. Severity classification (mild, moderate and severe)
There is no distinctive categorization for severity classification in this element. Hg concentration in whole blood below 20 μg/L is considered normal. This can rise to 35 μg/L after long-term exposure to Hg vapor. Hair analysis can present Hg exposure that has occurred over an extended period of time. Hair Hg concentration is a biomarker of long-term exposure to MeHg. Severe cases may be seen after acute exposure to methylmercury particularly in infants or children with neurological and GI symptoms, respiratory distress, and dermatitis mainly. The hair Hg concentration of more than 2400 mg/kg shall be expected. While pregnant mothers may have little or no manifestation of MeHg intoxication, exposed infants may face decreased birth weight and muscle tone, seizure, developmental delay, severe spasticity, blindness and deafness. In general, hair Hg concentration does not exceed 10 mg/kg. While in moderate mercury poisoning, the concentration level ranges between 200 and 800 mg/kg. WHO recommends MeHg evaluation in pregnant women's hair and warn that levels exceeding 10 ppm can elevate the risk of fetal neurological defects [39]. The health impact of Hg exposure is primarily focused on chronic, low or moderate grade exposure of MeHg.
3.1.6. Common treatment of mercury intoxication
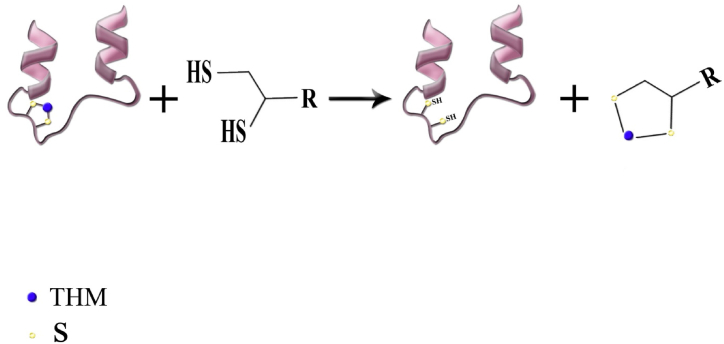
The first consideration in treatment of acute Hg exposure generally is removal of the patient from further exposure. Support of respiratory and cardiovascular function is also conducted. There is no specific antidote for mercury, but chelation therapy is favored in symptomatic patients. However, the effectiveness of therapy with chelating agents is uncertain in severely poisoned patients. Moreover, indications for the therapy have not been completely defined. Dimercaprol (British Anti-Lewisite, BAL), D-penicillamine, DMPS and succimer (meso-2,3- dimercaptosuccinic acid, DMSA) are among the chelating agents that can be utilized for the management of acute inorganic (Hg2+) and metallic (Hg0) mercury intoxication. The U.S. FDA has not approved any chelators for methylmercury or ethylmercury. BAL is contraindicated for the management of MeHg intoxication [39]. N-acetyl-D,L-penicillamine is an analog of D-penicillamine and has shown to be effective in Hg elimination from the body. The chelating drug is taken orally. It was claimed that N-acetyl-penicillamine poses similar efficacy and less side effects than D-penicillamine for the treatment of Hg poisoning [40,41].
3.1.7. Recent advances in the treatment of mercury intoxication
Shenkang, is a Chinese herbal medication which has been utilized in the clinical management of chronic kidney disease (CKD). The herb has exhibited therapeutic effects in Hg kidney sequalae [42]. Calcium carbonate, garlic tablet and N-acetyl cysteine (NAC) also gain popularity in some studies. Clinical trials are needed to study the effectiveness of new remedies [[43], [44], [45]]. Selenium may elevate distribution of Hg away from target tissue and increase Hg removal. Selenomethionine and sodium selenite are among selenium supplements being used for the management of Hg intoxication. Selenium can antagonize Hg at the molecular level via a few possible pathways; a more plausible one including Se-aided demethylation of MeHg [46]. NAC has shown the ability to bind with Hg. Moreover, NAC is a GSH precursor that can increase GSH production. GSH and many thiol (sulfhydryl, −SH) groups play a key role in defense mechanism against Hg toxicity [47]. Alpha-lipoic acid (α-LA), an organo-sulfur compound, can chelate Hg and some other THM by having two thiols in the structure. α-LA as a chelating agent that is able to penetrate the blood brain barrier (BBB) while other chelators such as succimer and DMPS cannot access to the BBB. α-LA can also regenerate antioxidants and hinder the free radical reactions [48]. Flavonoids has shown protective effect against kidney and liver injuries resulting from mercury chloride [49]. Myricetin, a flavonoid compound present in medicinal plants, has shown protective effects against mitochondrial dysfunction induced by MeHg in vitro by blocking oxidative stress and regulating inflammation [50].
To summarize, the treatment of Hg poisoning is an expanding field which is continually evolving. Many promising supplements according to recent studies like Shenkang, NAC, α-LA, and selenium show potential therapeutic applications. Clinical trials are indeed essential to validate their efficacy and establish effective treatment measures.
3.2. Lead (Pb)
3.2.1. Size of the problem and preventive measures against lead exposure
Exposure to lead and subsequent toxicity in humans occurs via ingestion, inhalation, dermal, and ocular contact; mainly by leaded paint and gasoline, adulterated opium and occupational environment [51]. According to the Occupational Safety and Health Administration (OSHA), the permissible exposure limit (PEL) for Pb is 50 μg per cubic meter of air on average over an 8-h shift [52]. One important source of Lead exposure in opium addicts is adulterated opium, which is now a matter of concern in Iran and other Middle Eastern countries that requires more governmental vigilance [51]. To reduce occupational exposure to Pb, adequate ventilation must be assured along with using personal protective equipment (particulate masks, gloves and goggles), avoiding dust formation and breathing vapors, and inspection for any leakage or spillage. Generally, the main step to prevent Pb exposure is to recognize and eradicate sources of Pb; e.g., paint in old buildings, lead-contaminated water and soil, car and industries exhaust and some toys [53].
Pb may induce oxidative stress via reducing antioxidant power, ROS generation, GSH depletion, mitochondrial damage, and inflammatory responses. In a rat model study, increased MDA level, decreased GSH concentration and activities of SOD and GST were underlying pathways of Pb-induced oxidative stress which were attenuated by grape seed extract. Besides, glycogen synthase kinase-3β was involved in the progress of oxidative stress in rat kidney induced by Pb. GSK-3β phosphorylates and inhibits Nrf2 pathway, contributing to Pb-induced renal damage [54]. Pb exposure can also have effect on gene expression of IL-6 and TGF-β1 cytokines. This can happen along with activation of phosphoinositide 3-kinase and p38 mitogen activated protein kinase [55,56]. Consequently, in response to IL-6 and IL-1, production of factors associated with inflammation are stimulated. C-Reactive Protein (CRP), an acute phase protein, along with cyclooxygenase 2 (COX-2) and lipoxygenase, play roles in inflammation, which can cause damage to microorganisms and cellular structures [57].
3.2.2. Acute lead poisoning
Lead poisoning, either acute or chronic, has been decreased during the past years due to improved vigilance of industrial and domestic exposures. However, it has not been eradicated completely. Although acute Pb toxicity is not common, it can still be induced following very high vapor inhalation, large ingestions and intravenous route. However, Pb-contained paint material and dust from old houses are considered the commonest sources of exposure. The clinical signs and symptoms usually consist of abdominal pain, hemolytic anemia, hepatic and pancreatic inflammations, and encephalopathy. When inhaled, Pb can cause cough, headache, confusion, drowsiness, metallic taste and even unconsciousness and convulsions. Eye contact leads to conjunctivitis [58].
Children have increased absorption ability of Pb and their classical manifestations mostly include neurological and GI symptoms and signs such as irritability, behavior and learning difficulties, abdominal pain, loss of appetite, vomiting and constipation. A body of evidence show that Pb affects the children's developing nervous system, thus, no safe blood lead concentration (BLC) is determined for children [58,59].
3.2.3. Chronic lead poisoning
The majority of poisonings induced by Pb are chronic. It is a silent disease that can attack multiple organs. Chronic poisoning may be either subclinical or not severe enough to present definite or readily detectable manifestations, or it might have a range of different symptoms in the neurological, GI, hematological, renal and cardiovascular systems. Inorganic Pb is now classified as a probable human carcinogen (Group 2B) and a confirmed animal carcinogen [60].
3.2.4. Prognosis for lead poisoning
The prognosis and outcome of lead poisoning are dependent on the duration and extent of exposure to this heavy metal. In children, lead poisoning can cause permanent health disorders, especially in the nervous system, that remains with them until adulthood. Higher levels of Pb in the blood can cause more severe and perhaps permanent damages; for instance, it can leave children with impaired intellectual ability and developmental problems [61]. Timely diagnosis and management of lead poisoning are critical since it can cause severe symptoms such as persistent GI and neurological disorders including encephalopathy [59]. Research have shown that exposure to Pb is associated with a higher chance of food allergies in adults.
3.2.5. Severity classification (mild, moderate and severe)
The severity of toxicity can be classified as mild, moderate and severe. Mild poisoning usually has non-specific manifestations that can only be detected by careful examinations and a relative elevation in BLC (20–69 μg/dL). Central nervous system (CNS) findings such as mood swings and psychiatric problems, tiredness, sleepiness and irritability are most dominant. Physical examination is either normal or mild hypertension may be detected. In moderate poisoning when BLC reaches 70–100 μg/dL, clinical symptoms and signs typically include CNS and peripheral nervous system (PNS), including headache, loss of memory, GI manifestations such as abdominal pain, loss of appetite, constipation, as well as the involvement of many other organ systems [62,63]. Albeit rare in adults, acute encephalopathy is the hallmark of severe toxicity and is diagnosed by seizures, confusion, papilledema, optic neuritis and headaches. Further effects of severe Pb toxicity can be detected as peripheral neuropathy, anemia, colicky abdominal pain, impaired kidney function and cardiac dysrhythmias. In this stage, BLC usually reaches above 100 μg/dL.
3.2.6. Common treatment of lead intoxication
Due to permanent and often life-threatening sequels of lead poisoning, timely treatment of symptomatic patients is critical. Irrespective of poisoning severity, the fundamental step of treatment is removing the lead sources or preventing further lead exposure whether occupational or non-occupational [51]. The next step is to determine the need for pharmacologic therapy with chelators and choose an appropriate method of treatment with respect to several factors such as clinical severity, BLC and each patient's individual factors. Currently, three chelating agents have been suggested for the treatment of lead poisoning; dimercaprol, calcium disodium ethylene diamine tetra acetic acid (CaNa2EDTA) and succimer. However, the only chelating agent that has U.S. FDA approval is succimer. It is used for the treatment of lead poisoning in children with BLC above 45 μg/dL. D-penicillamine, a chelating agent previously used for the treatment of lead poisoning, is now largely replaced by succimer, because of its significant adverse effects [64]. Overall, research has shown no proven benefit for administering chelators in patients with BLC 20–44 μg/dL [59].
3.2.7. Recent advances in the treatment of lead intoxication
Despite stationary regulations, lead exposure and poisoning are still globally observed, because of the wide application and environmental existence of Pb. This has led to research into different treatment regimens to potentially improve the management strategies [65]. Human research on the treatment of lead poisoning with different severities have focused more on the currently available chelating agents by putting them into different mix and match regimens with or without natural substances [63,66] while experimental studies are more directed toward finding novel treatment options, especially botanical plants and herbal medicines [[67], [68], [69], [70], [71]]. A recent retrospective study on 79 male adults with chronic Pb poisoning, the efficacy of succimer, as the standard treatment, with that of D-penicillamine (750–1500 mg/day) combined with garlic tablets (1500–3000 mg/day) were compared. Subsequently, clinical and laboratory data (BLC) were collected and assessed. The results indicated that treatment with D-penicillamine plus garlic yielded the same outcome as succimer with a significantly lower cost. The price for 30 days of treatment with D-penicillamine plus garlic was reported to be 28.6 times less than that of a 19-day regimen with succimer [63]. Molavi et al. [72] attempted to compare the clinical and laboratory efficacy of succimer vs. D-penicillamine in patients with acute lead poisoning over a 5-year descriptive study. Total of 163 patients received any of the three types of treatments: D-penicillamine, succimer, and D-penicillamine plus succimer. Research data were collected once on admission and then in 2 weeks. Analyses of data depicted that there was similarity in clinical improvement among the groups, hence reporting D-penicillamine as an acceptable chelator for acute Pb poisoning. Similar to Vahabzadeh et al. [63], the outcome of the study performed by the Molavi research team confirmed the advantage of D-penicillamine when access to succimer is limited. Additional empirical evidence supports the efficacy of D-penicillamine as a proper alternative in the treatment of Pb poisoning when there is a shortage of other chelating agents [63,66,72].
Since Pb exerts part of its toxicity by inducing oxidative stress in different tissues, natural antioxidants have been the subject of interest in many studies. Usman et al. [71,71] compared the effect of Moringa oleifera (250 mg/kg) vs. ascorbic acid (50 mg/kg) on biochemical, hematological and histopathological changes in a model of subchronic lead toxicity in male rats. The agents were administered for 6 weeks, after which laboratory analyses were carried out. The results showed some improvements in Pb-induced changes in tissues. Another study [69] in rats with Pb-induced liver toxicity, Moringa oleifera was shown to have anti-anemic, immune stimulant and liver protective effects by inhibiting oxidative stress, inflammation and apoptosis. Mohammadi et. al [70]. evaluated the antioxidant and chelating potential of silibinin and nano-silibinin in rats poisoned with Pb. Rats were exposed to Pb (50 mg/kg) with or without different doses of silibinin and nano-silibinin (25, 50 and 100 mg/kg) for six days, and then blood samples for BLC and biochemical antioxidant tests were collected. Silibinin, and more potently its nano form, could prevent weight loss, decrease BLC and normalize malondialdehyde and nitric oxide (lipid peroxidation products) to standard levels. Both antioxidants were effective in preventing oxidative stress in Pb poisoning [70]. Silymarin acts as an antioxidant through different pathways, hence making it an attractive substance for a lot of toxicities and diseases. Another study in rats compared liver protective effects of silymarin and D-penicillamine in acute Pb poisoning. Rats were exposed to Pb (25 mg/kg), D-penicillamine (100 mg/kg) and silymarin (200 mg/kg). Pretreatment with silymarin and administration of D-penicillamine significantly decreased hepatic transaminases and improved liver antioxidant enzymes [73]. Garlic is another herbal substance that has been the subject of interest for many clinical investigations. Scientific evidence exists for noticeable effects of garlic on Pb poisoning when prescribed either alone or in combination with pharmaceutical chelator agents. It was shown that garlic has the potential to decrease Pb from blood and tissues as well as to possess antimicrobial, hypolipidemic, anticancer, antioxidant and antithrombotic properties [63,74,75]. When compared to D-penicillamine in the treatment of patients with chronic lead poisoning, garlic appeared safer and as efficacious as D-penicillamine. It was administered in the amount of 6 cubes of fresh herb in one study [75], and in the form of a 400-mg tablet in another [74], with similar promising results. El-Khishin et al. [76] also studied the therapeutic effect of garlic (20 mg/kg/day) combined with silymarin (1000 mg/kg/day) in comparison with those of DMSA (30 mg/kg/day) in a mice model of lead poisoning. Results showed comparable efficacy of garlic plus silymarin with DMSA in amelioration of Pb-induced nephrotoxicity and improvement of the histopathological patterns [76].
An animal research from India investigated the potential protective effect of Linum usitatissimum (Flaxseed) and Emblica Officinalis (Amla) on renal and hepatic poisoning of Pb [77]. Experimental rats were received doses of 100 mg/rat/day Emblica Officinalis or 300 mg/kg Linum usitatissimum orally for 45 days, and afterwards, oxidative stress enzymes along with histopathological analyses were carried out. Results revealed that Flaxseed and Amla had protective effects against Pb-induced hepatic and renal toxicity with relatively superior potency for the Emblica in comparison with the Flaxseed. The antioxidant effect of Flaxseed was also evaluated in another [78] study on Pb-poisoned rats that received daily doses equal to 100 mg/kg of Flaxseed dissolved in 1 mL of lemon juice for one month. The lemon juice and Flaxseed could improve kidney and liver function tests, prevent lipid peroxidation and inhibit the reduction of glutathione (GSH) in kidney tissues. The positive effects of Ginkgo biloba to alleviate lipid peroxidation induced by Pb are well documented in laboratory animals. When administered orally in doses of 50 and 100 mg/kg for 14 days, Ginkgo biloba could improve hematological parameters, the kidney and liver function indices, and show antioxidant activity in rats [79]. In addition, further evidence from rodent models of Pb toxicity suggests neuroprotective effects for Ginkgo biloba by which it can decrease neuropsychiatric symptoms in this poisoning [80,81]. Thymoquinone (TQ) as a natural bioactive compound has shown therapeutic effects for many diseases due to its antioxidant properties. In a rat model of subchronic toxicity, TQ was able to attenuate testicular and spermotoxicity due to Pb exposure [82]. A derivative of lysine amino acid (abbreviated as HTPL) was promising for alleviating the subchronic lead toxicity. HTPL was administered orally similar efficacy to those of succimer and EDTA [83].
Recent experimental investigations describe the ability of probiotic microorganisms, micronutrients (such as zinc and vitamin C), and some herbal extracts (such as tea polyphenols and grape seed extract) to effectively control Pb toxicity [84]. The supplements could reduce Pb accumulation in tissues, decrease oxidative stress and improve the hematological and neurological dysfunction in mice with insignificant adverse effects. A literature review on such probiotics reported that four types of microorganisms were most protective against Pb-induced toxicity in pre-clinical research: three bacteria (Lactobacillus sp., Pediococcus pentosaceus, Bacillus sp.) and one yeast (Saccharomyces cerevisiae) [67]. Therefore, along with standard chelating agents and other supportive treatments, dietary probiotic supplementation may be considered as a promising approach to manage Pb toxicity. Further herbal extracts and natural substances have been evaluated with promising outcomes in animal models of Pb toxicity. Cinnamon [85] and thymus vulgaris extract (thyme) [68] have also been indicated to possess favorable effect on the alleviation of kidney and liver toxicity of Pb via antioxidant and immune-stimulant properties. Further studies are required to better elucidate the application of herbal substances and nutrients in human beings.
Vit D has shown protective effects against Pb-induced tissue damage in an animal model. The co-administration of Vit D with Pb could mitigate tissue injuries induced by the toxic metal exposure. The role of antioxidant and anti-inflammatory mechanisms can be significant in such protective effect [86]. Glycine has a similar protective effect in Pb-induced organ toxicity based on another animal study [87]. NAC has shown mitigating effect on Pb toxicity in the testicular tissues of male rats. It was found that NAC treatment could improve sperm quality and morphological changes, and modulate antioxidant balance and apoptosis in testis tissue [88].
In conclusion, although The US FDA approved only succimer as an antidote for Pb poisoning, other chelating agents including CaNa2EDTA and D-penicillamine can be used, particularly when succimer is not available or its high cost will not be affordable. CaNa2EDTA should prescribed as infusion for severe cases, and D-penicillamine for mild to moderate cases in combination with garlic tablets. Other antioxidants may be used as preventive medications or in combination with the above-mentioned treatments.
3.3. Chromium (Cr)
Chromium (Cr) is used in several industry such as chrome plating, stainless steel, paint pigments, leather tanning, and some heat-resistant applications. Cr toxicity mainly results from the environmental and or occupational exposures [89]. However, the acute toxicity of Cr induces the multi-organ failure. The chronic Cr toxicity is more related to cancers [11,90,91]. Cr has various oxidation states of −2 to +6, however, the trivalent (Cr3+) and hexavalent (Cr6+) forms are more common and the most relevant to human exposures [11] (Table 1).
3.3.1. Size of the problem and preventive measures against chromium exposure
Hierarchy of Controls is the main recommendation of National Institute for Occupational Safety and Health (NIOSH) for employers of Cr industries to consider the possible exposure for all staff and workers. For example; awareness of employers from Safety Data Sheet could reduce the Cr exposure and its hazard. The OSHA standards as average of 0.005 mg/m3 Cr (VI), 0.5 mg/m3 Cr (III), and 1.0 mg/m3 Cr (0) for an 8-h workday up to 40-h workweek. When employees expose more than 10 h per day, NIOSH recommends an exposure limit of 0.001 mg/m3 for Cr (VI) compounds in air. Air is another source of Cr exposure; people may be exposed to low levels of Cr by breathing its polluted air. The air of a city may be contaminated by Cr more than the rural or suburban air. In indoor air pollution by cigarette smoking, Cr concentration may reach to 10–400 times greater than the outdoor air concentrations. Thus reducing the smoking indoor air pollution could prevent the toxic effects of Cr. Agency for Toxic Substances and Disease Registry (ATSDR) suggests that total daily intake Cr from air should be less than 0.2–0.4 μg [92]. There is no recommendation for food or drug to reduce the absorption Cr or increase the Cr excretion. However, the oxidative stress may have an important role in reducing chronic Cr toxicity [11].
Markers of chromium (VI) exposure are generally biomarkers of oxidative stress, such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), malondialdehyde (MDA), and glutathione (GSH). Other promising indicators have been proposed for dealing with chrome, and more data are needed to confirm their significance. Cr may induce oxidative stress via ROS generation, reducing antioxidant capacity, depletion of GSH, mitochondrial damage, and inflammation responses [93]. Xu et al. (2017) used oxidative stress parameters and DNA damage biomarkers to evaluate markers of Cr exposure 319 participants living in Cr-contaminated areas and 307 unexposed participants. They found that serum concentration of MDA, CAT, GSH-Px, and 8-OHdG were elevated in the exposed population compared to the control group [94]. Another study reported on the Club (Clara) cell protein (CC16) an immunosuppressive protein, surfactant-associated protein D (SP-D), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) as biomarkers for lung injury in blood of 91 Cr occupationally exposed individuals and 38 controls. The findings expressed that the levels of SP-D, TNF-α, and IL-6 were higher and associated with Cr presence at workplaces [95].
3.3.2. Acute chromium poisoning
Cr6+ is a powerful oxidizing and caustic agent, thus massive Cr6+ ingestions can induce nausea, vomiting, diarrhea, vertigo, GI hemorrhage with or without bowel perforation, fever, muscle cramps, intravascular hemolysis with disseminated intravascular coagulation. Acute tubular necrosis leading to acute kidney injury, metabolic acidosis with elevated lactate concentration, hyperkalemia and uremia are consequences of acute massive exposure to Cr. Other presentations such as liver damage, acute multisystem organ failure, coma and even death were reported. Intoxicated patients are at high risk for acute respiratory distress syndrome (ARDS) up to 3 days. Cardiomyopathies and cardiovascular disease were reported after Cr inhalation [91]. Skin exposure to solutions contains Cr could induce penetrating lesions known as “chrome holes” or “chrome ulcers”, especially in injured epidermis. Exposure to 20 ppm Cr6+ may cause skin ulcers in unsensitized people. In severe dermal exposure, first and second degree burns that followed by delay onset GI and systemic symptoms may occur that resulted to death [96].
3.3.3. Chronic chromium poisoning
The most route of chronic Cr exposure is by inhalation. Inhaled Cr6+ irritates the respiratory tract and induces inflammation. Continuous Cr inhalation results in inflammation and nasal septum perforation, chronic cough, shortness of breath, occupational asthma, bronchospasm, keratitis, gingivitis and periodontitis. Pneumoconiosis is also reported in chromite miners secondary to chronic deposition of chromium dust. Chronic inhalation of Cr6+ is associated with an increased risk of lung and nasal cavity cancer after exposure for one decade. The most common types of lung cancer are small cell and poorly differentiated carcinomas. However, inhalation of Cr6+ may be associated with nearly all pathologic types of lung cancer. Chronic Cr6+ exposure causes mild to moderate raise in the hepatic aminotransferase enzymes and urinary β2-microglobulin concentrations [97,98]. Some epidemiology studies and meta-analysis revealed the positive relationship between Cr6+ exposure and gastric cancer in human as well as animal studies. However, previous meta-analysis could not prove the relationship [[99], [100], [101]]. Dermal contact to Cr6+ induces two types of dermatological toxicities: skin ulcers and allergic contact dermatitis. Frequent exposure to compounds contained 4–25 ppm of Cr6+ induces sensitization and elicit chromium allergic contact dermatitis. About 5 % of cement workers who exposed to 10–20 ppm Cr6+ for some years present chromium sensitivity [102]. Chronic skin contact with Cr such as cement workers develops skin sensitivity, and one fifth of Cr workers complain of contact dermatitis. A 33 years old woman who chronically ingested 6–12 times of the recommended daily dose of over-the-counter chromium picolinate (Cr3+) for 4–5 months, presented with weight loss, anemia, thrombocytopenia and hemolysis. Her abnormal lab tests were raised aminotransferase enzymes 15–20 times normal, raised total bilirubin 3 times normal, raised serum creatinine to 5.3 mg/dL and blood urea nitrogen 152 mg/dL. The plasma Cr level was 2–3 times normal [103].
3.3.4. Prognosis for chromium poisoning
The ingestion of high dose Cr (VI) compounds has poor prognosis and acute Cr poisoning could be fatal. The most frequent causes of death are large lesions in many tissues, GI hemorrhage and hypovolemic shock. If the patients survive, organ damage including acute liver and kidney failure may occur. Treatment is only symptomatic and using chelating agents have not been shown to be effective [104,105].
3.3.5. Severity classification (mild, moderate and severe)
There is no classification for Cr intoxication. Severe exposures to Cr6+ compounds are usually intentional (suicide) or accidental, and are rarely occupational or environmental. The average human oral lethal dose of Cr6+ is estimated as 1–3 g.
3.3.6. Common treatment of chromium intoxication
As for the other toxicologic emergencies, the basic supportive care of airway, breathing and circulatory should be done at the first step, and then decontamination shall be applied if required. Cr3+ compounds need limited decontamination with soap and water in case of skin contact. No pulmonary or GI decontamination is recommended [106]. In contrast, Cr6+ gastric lavage by nasogastric tube may be considered in a patient presents within a few hours after ingestion with no vomiting. However, nasogastric tube insertion is an aggressive procedure and may not be indicated in injured esophagus gastroscopy may be indicated. There is no recommendation for administration of activated charcoal. There are some case reports, that hemofiltration, hemodialysis and peritoneal dialysis were applied in Cr poisoning. Hemofiltration did efficiently remove Cr. Thus, it seems that peritoneal dialysis or hemodialysis is a reasonable therapeutic choice in the setting of acute or chronic kidney dysfunction due to Cr intoxication [107,108].
One of the main parts of metal poisoning treatment is chelating therapy. There are several suggested chelators such as BAL, EDTA, D-penicilamine, succimer and DMPS. However, there is little evidence for the effect of known chelators in Cr intoxication and there is no approved chelator for that [40,109]. EDTA was reported of not being effective for the management of Cr intoxication. Treatment of 16 patients with EDTA significantly raised the urinary excretion of lead, zinc, cadmium and calcium, but did not significantly change urine Cr level [110,111]. Anderson et al. [112] also reported an evaluated urinary Cr loss by various EDTA treatment regimes in two groups of human subjects who had taken Cr-supplementary. They found no relationship between urinary Cr and EDTA treatment [112]. Iron chelators have shown effective outcome in the treatment of Cr intoxication. Deferoxamine pretreatment in rat prevents nephrotoxicity by Cr6+ through lowering the oxidative stress or a chelating mechanism [113]. Iranmanesh et al. [114] evaluated the effect of single and a combination of deferasirox and deferiprone on Cr level of liver, kidney, intestine, spleen and testicle of chronic Cr6+ intoxicated rats. Rats received two doses of 15 and 30 mg/kg Cr6+ for 60 days. The Cr tissue levels of both groups were reduced by treatment with each of iron chelators, and combination of them was more potent. However, there is no evidence for these effects in humans [114].
3.3.7. Recent advances in the treatment of chromium intoxication
Reactive oxygen species (ROS)-mediated oxidative stress is known as one important mechanism of Cr toxicity. Thus Cr-induced damages may be alleviated by agents that restore antioxidant balance [91]. NAC is a potent antioxidant that was applied in acetaminophen [115], lead [116], phosphine [117], paraquate [118], and THM poisoning [119]. Cr6+ induces oxidative stress and decreases GSH level. Thus, supplementary NAC could ameliorate the damage from Cr-induced oxidative stress [120]. Chelating is another suggested mechanism of antidotal effect of NAC against Cr. NAC could effectively excrete Cr through urine in potassium dichromate intoxicated rats. NAC also reversed oliguria in animal model. Moreover, NAC has also a chemoprotection mechanism by losing Cr6+ accumulation in cells through reduction of chromate to membrane-impermeable Cr3+ [121]. Increasing ROS, release of inflammatory cytokines and autophagy are important suggested mechanisms of skin adverse effects of Cr–induced allergic contact dermatitis. NAC can inhibit the expression of IL-1α and TNF-α mRNA induced by Cr (VI) in HaCaT cells [122]. Severity of intradermal and epicutaneous elicitation test of Cr-treated albino guinea pigs was reduced when the animals were treated by 1200 mg⁄kg⁄day of NAC. Sensitization rate of Cr hypersensitivity was also reduced by the same dose of NAC [123]. Pretreatment of Cr intoxicated HaCaT cells with NAC reduced apoptosis and autophagy and raised cell viability. Reduction in Cr cytotoxicity by NAC may be time dependent. Treatment of Cr intoxicated human proximal tubular epithelial cell line HK-2, with 600 or 1000 μg/mL NAC within 2 h reduced the expression of intracellular ROS and protected the kidney cell line from Cr6+-induced cytotoxicity. But 4 h and 8 h delayed treatment with NAC did not shown significant results [122]. NAC also improved the impaired glycemic state and enhanced insulin secretion and antioxidant competence in pancreatic beta-cells of Cr intoxicated rats [124].
A case study, reported a 47-year-old man who developed hepatic injury due to ingestion of 30 mL of plating solution. His liver function tests were improved after treatment by NAC and ascorbic acid. He was treated by 100 mg of intravenous ascorbic acid q8h and 1200 mg q12h orally NAC from 5th up to 13th day of hospitalization. However, his clinical conditions, general malaise, jaundice and total bilirubin level worsened again after discontinuing these therapies and then gradually improved [125]. Two cases of metal-on-metal hip-implanted patients treated by NAC were also reported. They were 67-year-old male and 81-year-old female who underwent Co/Cr hip implant and high Cr levels were detected in their serum. Serum Cr levels were reduced 87 % and 24 % after 300 mg/kg/day of NAC for 9 and 10 days, respectively [126]. Another case study reported an electroplating worker with severe Cr intoxication who developed multiorgan failure after skin exposure. His legs immersed in the electroplating solution that induced a chemical burn of 15 % of the body surface. He had been treated by intravenous DMPS 125 mg every 12 h, intravenous NAC 50 mg/kg every 4 h and ascorbic acid 100 mg every 12 h since the day after exposure. He also performed continuous veno-venous hemofiltration and plasmapheresis since 3rd day of hospitalization due to renal failure and thrombocytopenia. NAC, vitamin C, DMPS and plasmapheresis were discontinued at 10th day, however hemodialysis continued up to 30th day. He was completely recovered 3 months later [127].
Liver of chicken treated by Cr6+ showed increased malondialdehyde (MDA) content and decreased GSH content, Ca2+-ATPase activity, and mitochondrial membrane potential (MMP) level. Selenium supplementation could reverse Cr toxicity in chicken and alleviated Cr induced hepatic injuries in moderate dose [128]. In sub-acute and chronic animal models of Cr toxicity, melatonin; a powerful antioxidant; prevented hepatic enzymatic and non-enzymatic antioxidants reduction. Melatonin also reduced raised lipid peroxidation level and revealed liver histopathological changes [129]. The females first generation of rats; whom fed by their mother's milk and their mothers were treated by potassium dichromate through postpartum days 1–21; showed follicles atresia, raised cytochrome C and cleaved caspase-3, reduced antiapoptotic proteins, raised metabolic clearance of estradiol with reduced estradiol biosynthesis and impaired oxidant/antioxidant balance. Resveratrol (10 mg/kg body wt., through oral gavage daily), an antioxidant of grabs, showed a protective effect against toxic effects of Cr on ovary [130]. Membrane damage is considered an important mechanism of Cr cytotoxicity. Since alpha-tocopherol, a membrane protectant reduced this damage, it has been suggested that dietary supplementation of alpha-tocopherol may restrain the Cr-induced membrane damage [131].
Briefly, several promising approaches emerging from recent studies seems favorable in the treatment of Cr intoxication. NAC, selenium, melatonin, resveratrol, and alpha-tocopherol offer additional benefits for mitigating Cr-induced intoxication. Despite the encouraging findings, further clinical trials and mechanistic studies are essential to establish effectiveness of such treatment protocols.
3.4. Cadmium (Cd)
3.4.1. Size of the problem and preventive measures against cadmium exposure
The major route of exposure to Cd is air, water, soil and food. Upon entering to the body, Cd exerts its toxic effects through different mechanisms [11]. It is emitted to the air from the weathering of rocks, forest fires and volcanic eruptions [132]. In addition, Cd accumulates in the environment resulting from industrial production including mining, smelting, battery manufacturing, fossil fuel combustion, Cd plating and chemical industry, fertilizer manufacturing and waste disposal [133,134].
Tobacco smoke is the major source of Cd atmospheric pollution and a serious health problem. The tobacco leaves contain one and two μg Cd in each gram dry weight of leaves. Therefore, each cigarette may have 0.5–1 μg Cd. The absorption rate of Cd from lung is much higher than the intestine. As a result, blood Cd concentrations may reach to 5 times higher in tobacco smokers in comparison with nonsmokers [135].
Apart from smokers and occupational population, food is the main environmental source of Cd exposure [[136], [137], [138]]. Cd pollution of aquatic environment is caused by release of pollutants from the air, metal smelters, coal combustion and the disposal of sewage sludge. In addition, atmospheric deposition, metal smelting, fuel combustion, sewage sludge and phosphate fertilizer are main sources of Cd contamination in soil. Cd may have long-term damaging effect on agricultural soils. Cd in the soil is transferred to plant and accumulates in agricultural products in large quantities. Because of elevated mobilization potential of Cd, it can easily enter the food chain.
The following strategies can be effective in controlling and reducing exposure to Cd and existing health hazards:
-
I.
In the workplace with possible exposure with Cd, occupational hygiene and improved ventilation should be considered as the first line of defense.
-
II.
The use of Cd and its compounds as stabilizers and pigments in plastics and paints or in packaging materials should be limited or banned.
-
III.
Nickel-Cd batteries and accumulators containing with higher than allowable limit of Cd must be labelled and recycled or disposed properly.
-
IV.
Production and smoking of cigarettes containing Cd must be limited.
-
V.
Individuals should have adequate iron, zinc, and Se in the diet, because those who are at risk for micronutrients deficiency may absorb more oral Cd.
-
VI.
The use of phosphate fertilizers in agriculture sector should be avoided.
-
VII.
Every effort should be made to maintain the permissible level of Cd in air, drinking water, soil or food.
-
VIII.
Natural and chemical substances can be applied to reduce the environmental Cd contamination. The seeds of some medical plants like peanuts (Arachis hypogaea) were suggested for water purification. Phytoremediation can also be used to extract and detoxify elemental pollutants in soil [139,140].
β-2-microglobulin (B2-MG) and N-acetyl-β-D-glucosaminidase (NAG) are well-established, sensitive, and the most common effect biomarkers to relate Cd or Cr exposure to renal tubular dysfunction. α-1-microglobulin in urine is another sensitive biomarker for Cd exposure implying proteinuria due to renal tubular damage [141,142].
3.4.2. Acute cadmium poisoning
Acute Cd poisoning is characterized by respiratory irritation and occurs as result from inhalation exposure to Cd oxide. Within 6–12 h of exposure in a closed space, the patient manifests symptoms including cough, fever, and or respiratory distress or trouble breathing. Cd pneumonitis may progress to hypoxia, pulmonary insufficiency, or even death. Patients usually appear well and have a normal physical examination, oxygen levels, and chest X-ray on initial presentation. Cd pneumonitis can progress, with symptoms of rhonchi, impaired oxygenation and alveolar infiltrates on a chest radiograph. In severe cases, death often occurs within 3–5 days of exposure. Patients, who experienced pneumonia after an acute Cd poisoning, are at higher risk of pulmonary disorders. Generally, acute inhalation of Cd is more likely to occur than acute ingestion. Cd ingestion in high quantity causes symptoms like food poisoning.
3.4.3. Chronic cadmium poisoning
3.4.3.1. Nephrotoxicity
Cd accumulates predominantly in the kidneys. The hallmark feature of renal damage in chronic Cd toxicity is proteinuria that is generally irreversible even after exposure removal. Other early signs of injury may include glomerular dysfunction, calcium loss and tubular lesion. In severe cases, glycosuria, hypercalciuria, hypophosphaturia, polyuria aminoaciduria, and decreased buffering capacity may be manifested [139,143].
3.4.3.2. Pulmonary Toxicity
The incidence and/or severity of Cd pulmonary toxicity depend on dose and duration of exposure. Some evidence indicated on a possible involvement of Cd in lung injury such as chronic obstructive disease and emphysema. Improvement in pulmonary symptoms has been reported after removal from Cd exposure [143].
3.4.3.3. Musculoskeletal Toxicity
Environmental Cd exposure can be a cause of osteotoxic effect. Age, sex, cumulative dosing and route of exposure are the reason of appearance of osteomalacia in Itai-itai victims (old women) and in patients with environmental exposures. The Itai-Itai patients experienced a broad range of signs and symptoms, such as a low bone mineralization, bone decalcification, a high incidence of fractures and distortion of the long bones, intense bone pain, osteomalacia, and osteoporosis. Cd exposure may promote the development of musculoskeletal health problems, such as osteoarthritis and rheumatoid arthritis [144].
3.4.3.4. Other Organ systems
Cd poisoning can cause nerve damage to the olfactory nerve and cortical function and lead to development of Parkinsonism [145,146]. Cd exposure is also associated with increased risk of atherosclerosis and heart failure [147]. Indeed, Cd can cause a deficiency of testosterone production leading cell hyperplasia [148]. Hepatotoxic and immunosuppressive effects are not prominent features in humans with Cd exposure.
3.4.3.5. Carcinogenicity and Cancer burden
Cd compounds are categorized as carcinogenic and recognized as a carcinogen in humans by different regulatory agencies. The International Agency for Research on Cancer (IARC) classified Cd as Group 1, carcinogenic to humans [149]. Epidemiological surveys suggest that occupational Cd exposure is associated with an increased risk of lung cancer. Some reports suggested that Cd might be involved in prostate, genitourinary, breast and renal cancer, although this linkage is weaker than for lung cancer. Furthermore, Cd has also been linked to malignancies of liver, pancreas, stomach, thyroid and hematopoietic system [143,144,150,151].
3.4.4. Prognosis for cadmium poisoning
The prognosis for Cd toxicity depends on many factors including route of exposure, dose, and duration of exposure, rapid detection of Cd in blood samples, severity of clinical manifestation and initial management of poisoning. Patients with severe intoxication may need more aggressive treatment.
In acute Cd poisoning, death may occur due to respiratory irritation following inhalation exposure. The inhalation exposure to 5 mg/m3 for 8 h may be lethal. The ingestion lethal dose is estimated in the range of upward from 150 mg.
In chronic exposure, various organs can be affected including kidney, lung, musculoskeletal and nervous system. Cd exposure can be associated with early signs of renal dysfunction, proteinuria, calcium loss and tubular lesion. Cd has a long half-life in the kidney and therefore it would be reversed if it is detected in lower body burden. In patients with pneumonia, aggressive treatment should be considered to prevent of persistent restrictive lung disease [152].
3.4.5. Severity classification (mild, moderate and severe)
There is no classification for severity of Cd toxicity. Severe toxicity can occur through accidental or intentional (suicide) poisoning [143,153]. The lowest human's lethal dose of Cd is estimated as near 70–80 mg/kg.
3.4.6. Common treatment of cadmium intoxication
Immediate considerations focus on GI decontamination and supportive care. Acute poisoning due to large ingestion of Cd compounds is rare, but it may be fatal. If vomiting has not occurred spontaneously, gastric lavage is recommended soon. Pulmonary damage is suspected in patients with exposure to Cd fumes. Corticosteroids are used to reduce the inflammation and control the respiratory complications. All patients with evidence consistent with Cd exposure should be hospitalized and evaluated for renal, hepatic, GI, urinary and respiratory tracts injury and function [144]. Hemodialysis is not effective in treatment of Cd intoxication and it is indicated if the renal failure is present [143].
Although using chelators is the preferred medical treatment in acute ingestion of Cd, the benefit of chelation therapy remains unproven. Among different chelating agents, succimer can be assumed as an effective drug due to decreasing the GI absorption without accumulation in target organ and improve survival. Other chelators with possible benefits include DMPS, diethylenetriaminepentaacetic acid (DTPA). Although dimercaprol (BAL) is a powerful chelator for Cd as other THM metals, it is reported that the Cd-BAL complex is much more nephrotoxic than Cd itself [154]. Thus, dimercaprol increases the Cd of liver and kidney and it is not recommended to give in Cd intoxication [143]. It has been reported that the other chelators could be either ineffective or detrimental as for penicillamine, tripolyphosphate, nitrilotriacetic acid, CaNa2EDTA and dithiocarbamates [155].
3.4.7. Recent advances in the treatment of cadmium intoxication
Variety of preventive and treatment approaches based on underlying mechanisms against Cd toxic effects have been studied in recent years. Natural compounds with antioxidant and anti-inflammatory properties such as curcumin, ginger, resveratrol, physalis extract, grapefruit juice, allicin, royal jelly, spirulina, quercetin, caffeic acid phenethyl ester, vit C, vit E, NAC, coenzyme Q10, alpha-tocopherol, selenium and zinc may be good candidates to reduce Cd poisoning [[156], [157], [158], [159]]. N-benzyl-D-glucamine dithiocarbamate (BGD), N-tetramethylene dithiocarbamate (ATC) and N-p-hydroxymethylbenzyl-D-glucamine dithiocarbamate (HBGD) have shown protective effect against Cd cytotoxicity by decreasing the Cd level (increasing the urinary excretion) and oxygen species activity [[160], [161], [162]]. Royal jelly from worker honey bees, showed protective effect against Cd-induced nephrotoxicity in mice. The observed reno-protective effect makes this natural compound a potent candidate for renal protection against Cd toxicity [163]. A combination therapy of DMSA combined with its analog MiADMSA, which is not yet commercially available, has been claimed to mobilize intracellular Cd deposits to some extent via its ability to cross biomembranes [164].
The results of in vitro and in vivo studies indicated that the probiotic microorganisms are able to protect against acute and chronic Cd toxicity mainly by reduction of the intestinal Cd absorption; decreasing the Cd accumulation in the tissue and alleviating the oxidative stress [165]. It also has been reported that essential trace elements such as zinc, selenium, or iron can be used for the susceptible population to long-term exposure to Cd [155].
In conclusion, the treatment of Cd intoxication is an evolving field, with various natural compounds and chelating agents showing promise in mitigating Cd toxicity. Natural antioxidants like curcumin, ginger, and royal jelly, along with established chelators such as DMSA, show viable therapeutic options. Furthermore, the role of probiotics and essential trace elements in reducing Cd exposure highlights the complex approach necessary for effective management. Future research should focus on clinical trials to validate the efficacy of these treatments.
3.5. Arsenic (As)
3.5.1. Size of the problem and preventive measures against arsenic exposure
The Environmental Protection Agency (EPA) and WHO set the safe contaminant level of As in drinking water to be less than 10 ppb (0.010 mg/L) [166,167]. In the workplace, the limit set by the OHSA is 10 μg (μg) of As per cubic meter of air for 8-h shifts and 40-h weeks. One of the strategies for prevention of exposure to As is providing a safe water supply for drinking, irrigation of food crops and food preparation. As contaminated soils are remediated by mycoremediation, amalgamating various bioremediation techniques and phytoremediation [168,169]. Also, human may be exposed through food with pesticides residues grown in As rich soil or irrigated with contaminated water. There may be As in some foods, including meat, poultry, fish, and rice. Rice being the food for many people, significantly contributes more As than any other food source. One of the prevention techniques is the adaptation of cooking and irrigation ways for rice. Using genetically modified rice that would accumulate less As within the rice grains is another strategy [[170], [171], [172]]. Nutritional status may play a role in preventing As toxicity. Diet rich in selenium, zinc and other antioxidants (such as vitamin E, vitamin C), and folate help to decrease As toxicity. Research on the potential protective roles of thiol and selenol compounds, including new chelating agents against As toxicity are of great importance in the future [[173], [174], [175], [176], [177]].
Oxidative damage markers for lipids such as malondialdehyde (MDA) as main lipid peroxidation product, proteins (e.g., carbonylation), and nucleic acids such as 8-hydroxydeoxyguanosine provide a more accurate representation of oxidative stress and its biological implications due to As induced damage. As can induce both eustress and distress conditions in many organs and tissues [178,179].
3.5.2. Acute arsenic poisoning
Arsenic has two chemical valences of three and five. The three-valence compound (As2O3) is more toxic than the five-valence compounds. There are variations in different people, population groups and geographic areas for As exposure [11,180,181].
3.5.2.1. Acute toxicity
Acute As poisoning occurs more commonly from accidental ingestion, homicide or suicide and infrequently from the workplace. Acute toxicity presents with gastroenteritis followed by hypotension. Gastroenteritis manifestations include nausea, vomiting, diarrhea, abdominal pain, garlic odor in breath and body tissues, hypersalivation, thirst, dehydration, and dysphagia. Symptoms begin within 30 min to 2 h after ingestion and resolve within 12 h, but may present for days after exposure. The cholera-like or rice water diarrhea is the classic type of diarrhea which may also be bloody. Arsenate (pentavalent form of As) is corrosive and may cause oral burns, dysphagia and GI bleeding [180,181]. Hypotension secondary to dehydration and volume loss is the most important cardiovascular manifestations. Electrocardiogram (ECG) abnormalities may be observed hours to days after exposure including prolonged QT interval, QRS lengthening, non-specific ST-segment changes, T wave flattening, torsade de pointes and ventricular fibrillation [182]. Neurologic symptoms including delirium, tremor, headache, weakness, leg/muscular cramps, encephalopathy, hyperpyrexia, seizures, autonomic neuropathy with anhidrosis, sweating, flushing and unstable blood pressure have been reported [11]. Pulmonary involvement may present as cough, dyspnea, and chest pain which is more commonly seen with inhalation exposure. In severe cases, non-cardiogenic pulmonary edema, adult respiratory distress syndrome and acute respiratory failure can be present. Proteinuria, glycosuria, leukocyturia, hematuria, and acute tubular necrosis with oliguria have been reported hours to days after exposure. Hepatic involvement including elevated liver enzymes, jaundice, pancreatitis, fatty infiltration, congestion, central necrosis, cholangitis, and cholecystitis; and hematologic abnormality such as anemia, thrombocytopenia, leukopenia, bone marrow suppression, acute hemolysis and disseminated intravascular coagulation are another presentation of acute poisoning. Other reported manifestations include skin rashes, rhabdomyolysis and conjunctivitis [11]. As can cross the placenta and is teratogenic. Fetal demise due to the toxic As concentrations in the fetal organs has been reported [183]. Patients with acute toxicity should be monitored for at least four to 6 h after suspected ingestion. Spot urinary As concentration; As levels in 24-h urine; chest x-ray and plain abdominal X-ray is requested for patients with acute poisoning. Organic As compounds present in the urine may be from food sources. Therefore, the patient should be asked if shellfish was recently eaten. Hair samples may be positive 30 h post exposure. They cannot differentiate between ingestion and external exposure. Serum As levels are not a reliable marker for As toxicity because of rapid As clearance from the bloodstream [[184], [185], [186]].
Arsine gas (AsH3) is an extremely toxic form of As compounds. Inhalation of arsine gas above 10 ppm is lethal. Arsine gas is colorless and odorless that does not cause tissue irritation. It is a hemolytic agent. The symptoms appear very soon after exposure. Hemolysis with hematuria and jaundice can last for several days [180,187,188]. Symptoms include garlic breath odor, nausea, and vomiting, abdominal pain, diarrhea, headache, chest pain, imbalance, tachycardia, fever, hematuria, liver function disturbance. Renal failure 1–12 h after exposure up to 24 h after acute exposure may be observed. Port wine colored urine, reddish conjunctiva, and jaundice reported up to 48 h after exposure. High peaked t-waves, conduction abnormality, heart blocks, and asystole may be observed. Cardiac and renal failures are commonly the causes of As-induced death [181,187,188].
3.5.2.2. Subacute toxicity (1–3 weeks)
Survivors of acute As poisoning and patients who are slowly intoxicated environmentally may manifest sub-acute toxicity. One to three weeks after exposure to As, some clinical manifestations may occur as following:
Neurologic disorders are initially sensory which may begin 2–4 weeks after resolution of the initial signs of acute As poisoning. Weakness, loss of deep tendon reflexes, gait disturbances, temperature sensation, vibratory sensation and quadriplegia reported in severe cases, and in rare instances, respiratory muscle insufficiency. There are also reports of headaches, confusion, memory loss, seizures and delirium. Leukopenia, thrombocytopenia, basophilic stippling and anemia, hypotension, hemolytic vomiting, diarrhea, abdominal pain, difficulty in swallowing, severe hypovolemia, cardiomyopathy, ventricular dysrhythmias and congestive heart failure are other clinical manifestations [180,181].
3.5.3. Chronic arsenic poisoning
Chronic As toxicity is associated with various clinical manifestations known as arsenicosis [167]. Peripheral neuropathy and skin lesions are the most common effects of chronic As poisoning. ([[189], [190], [191], [192], [193]], Wang, Karvonen-Gutierrez et al., 2022, [194]). Skin lesions are hyperpigmentation on the face, neck and back and a “raindrop” appearance on the trunk and neck; hyperkeratosis of the palms of the hands and soles of the feet. Skin manifestations are not commonly seen in As inhalation exposures. Mees lines as 1–2 mm wide transverse white bands across the fingernails presents 4–6 weeks after exposure. Dermatitis, melanosis, vesiculation warts, alopecia, conjunctivitis and corneal ulceration are other manifestations [167,192]. Peripheral neuropathy, motor paralysis, specifically of the distal extremities, hearing loss, encephalopathy, symmetrical peripheral polyneuropathy, muscle fasciculation and ataxia are the other manifestations of chronic toxicity [167]. Cardiovasuclar toxicity includes carotid atherosclerosis, hypertension, arrhythmias, pericarditis, Raynaud's syndrome, acrocyanosis (intermittent), ischemic heart disease, black foot disease, and cerebral infarction due to microcirculation abnormalities [167,191]. GI effects may be observed as anorexia, abdominal pain, diarrhea, weight loss, esophagitis, gastritis, and colitis. Liver toxicity present with increased hepatic enzymes with enlarged and tender liver, jaundice, cirrhosis, fatty degeneration, portal hypertension without cirrhosis, ascites and angiosarcoma. Anemia, leukopenia, thrombocytopenia due to bone marrow hypoplasia, impaired folate metabolism and karyorrhexis are hematologic features. As might influence the risk of metabolic syndrome in midlife women [195]. It is also related to low working memory in adolescents [196,197]. As has been classified as group 1 human carcinogens and the related toxicity may lead to carcinogenicity and mutagenicity. It elevates the risk of the skin, kidney, bladder, liver, lung and breast cancer [189,193].
3.5.4. Prognosis for arsenic poisoning
Prognosis of As poisoning correlate to the toxicity severity which depends on combination of several factors including age, As state, route of exposure, dose, duration of exposure, underlying diseases, early detection of As in biological samples and the availability of chelators. In acute As poisoning, death is usually due to hypovolemic shock and cardiovascular collapse. The minimal lethal dose is in the range of 1–3 mg/kg. The estimated lethal exposure dose of inorganic As is 0.6 mg/kg. Lethal doses can result in death within 1–4 days after ingestion [181,198]. Survivors of an acute poisoning will mostly develop neural injury (sensory and motor disturbances). They may have been cardiac, renal, liver and skin problems and the prognosis may be poor. Recovery from chronic As poisoning may take long time and may not be completed especially for peripheral neuropathy [181,198].
3.5.5. Severity classification (mild, moderate and severe)
There is no specific severity classification for As toxicity. Toxicity can be acute, sub-acute and chronic. Severity of poisoning depends on several factors including the type of As compound, valence state, toxicity dose, route of exposure, duration of exposure and underlying diseases. Other factors include individual variations in methylation and excretion [11].
3.5.6. Common treatment of arsenic intoxication
3.5.6.1. Acute As poisoning
Aggressive intravenous fluid and electrolyte replacement therapy is lifesaving in severe poisoning. However, maintaining the fluid balance is important because of cerebral and pulmonary edema which may be occurring. GI decontamination is controversial. Although As is poorly adsorbed to activated charcoal, it is performed because of low likelihood of harming the patient. It is also administered in the case of co-ingestion. In case of radiopaque-material visualization in the GI tract, bowel irrigation is recommended until it is no longer visualized on repeated radiographs. Continuing nasogastric suction removes As re-secreted in the gastric or biliary tract [199]. Hemodialysis should be a consideration for patients with acute kidney injury (AKI), oliguria and or renal failure. Also, washing with soap and water and rinsing affected skin, antiemetic, blood transfusion (in case of hemolysis), using mineral supplements that lower the risk of potentially fatal heart rhythm problems are other modalities. Treatment with magnesium sulfate, amiodarone, lidocaine and defibrillation if ventricular tachycardia or torsades de point occurs, is suggested. The use of IA, IC and III antiarrhythmics secondary to risk QT prolongation should be avoided. Seizures are treated with benzodiazepine and general anesthesia if necessary [199,200]. Succimer, BAL and DMPS are the most important used chelators. The goal of chelation therapy is to achieve a 24-h urinary As level below 50 μg/L. The chelators are most effective within minutes to hours post exposure [[201], [202], [203]].
3.5.6.2. Arsine gas poisoning
Treatment primarily involves supportive care and is aimed at preserving renal function. Red cell transfusion, folate or iron supplementation is necessary for the patients with significant hemolysis. Hemodialysis should be considered for patients with oliguria, AKI or renal failure. Exchange transfusion removes non-dialyzable arsine ([199], Wang, Karvonen-Gutierrez et al., 2022).
3.5.6.3. Chronic As poisoning
Treatment is mostly symptomatic. It involves primarily on the identification and elimination of the toxic source as well as to provide supportive measures. Stoppage of drinking of As-contaminated water is the mainstay of the management. Workers who are exposed occupationally to As may express their concerns with employers. After an intervention, follow-up urine and clinical testing may be applied to verify a reduction in exposure [199,200]. Chelation has limited efficacy in chronic exposures. In cases of sub-acute and chronic toxicity, it is recommended to await laboratory confirmation prior to beginning chelation therapy, unless there is a worsening of the patient's clinical condition. Succimer is the preferred chelator for sub-acute and chronic toxicity. Liver function tests and metal level assessment should be monitored in patients needing prolonged treatment.
3.5.7. Recent advances in the treatment of arsenic intoxication
An analog of succimer, monoisoamyl ester, have increased survival in rats poisoned by As [204,205]. A chelating agent monoisoamyl 2,3-dimercaptosuccinic acid (monoisoamyl DMSA, MiADMSA) is being currently in clinical phase I study for the treatment of chronic As poisoning [206]. Increased antioxidant capacity of cells could reduce As toxicity. Montelukast was effective at reducing As-induced epithelial-mesenchymal transition in bronchial epithelial cells [207]. An animal model revealed that NAC has a protective effect against As-induced liver injury [207]. Melatonin had also a significant effect in reducing age-dependent cytotoxicity of As in rats' brain [208]. In an in vitro study, green tea (Camellia sinensis) showed a preventive effect in As oxidant stress [209,210]. Polyphenol-rich apple (Malus domestica) peel extract ameliorate the cardiotoxicity of As trioxide in h9c2 cells [211]. Spirulina (Cyanobacteria) extract removed As from isolated liver cells [212]. Moreover, virgin coconut oil (VCO) protected hepato-renal damage against As-induced oxidative stress [213]. The antagonism effect of ginger against As-induced hepato-renal poisoning has been evaluated in a study by Panda et al. [214]. Ginger powder effectively counteracted the toxic effects of As in ducks [214]. Kumar et al. [215] revealed the protective efficacy of Coriandrum sativum seeds extract against toxicity of sodium arsenite in mice [215]. Coriandrum sativum seeds extract had ameliorative effect against liver and kidney toxicity induced by As. In another study, Aspalathus linearis ameliorated tissue toxicity associated with As exposure in rats [216]. In vivo studies have been shown that Viscum album and Phyllanthus emblica leaves, Syzygium cumini leaves, Moringa oleifera leaves and Ipomea aquatica aerial parts had ameliorative effects in As toxicity. Flavonolignan (silibinin), Allium sativum, Curcuma longa, Silybum marianum and some fibers and algae were the effective herbals for the treatment of As intoxication [217]. The intake of zinc [218,219] and selenium [[220], [221], [222]] may reduce As-induced toxicity. An inverse relationship has been shown between dietary intake of folate [[223], [224], [225], [226]], pyridoxine and riboflavin [227], vitamins A [228], C [174,229,230] and E [[231], [232], [233]] with the severity of skin lesions in As toxicity. However, another study did not find a statistically significant relationship between the supplementation with vitamin E, selenium, or both and the severity of skin lesions [234]. Inter-individual susceptibility plays an important role in As toxicity. Variation in As biotransformation, genetic, environmental and physiological factors including lifestyle and nutritional status, other diseases, and co-exposure toward other THM may regulate the extent of As-induced skin lesion [235,236]. Clinical trial studies have shown the usefulness of vitamins A, C and E in arsenicosis patients [237]. Nutritional supplementation is controversial in the absence of dietary deficiency. More researches are needed. Natural compounds including β-carotene, α-tocopherol, genistein, α-LA, eriodictyol, hydroxytyrosol, epigallocatechin-3-gallate, oleuropein, ellagic acid, rutin, curcumin, quercetin, naringenin, sulforaphane, allicin, lutein, biochanin, resveratrol and D-pinitol have been alleviated As toxicity in some animal models and in vitro studies [217]. Garlic oil has been effective in the keratosis induced by As [238]. Co-administration of phytochemicals with a chelating agent eliminated more As from the body with less adverse effects because of a reduced dose of chelating agent [239]. Chelating agents such as MiADMSA when administered along with curcumin as a phytochemical or herbal extract have been shown to be effective [240]. It has been suggested that nutritional combination therapy is more beneficial for the treatment of the chronic As exposure than usual chelation monotherapy. However, clinical study is needed to accurately determine the dose. Proteins, amino acids, flavonoids, polyphenols, jiggery containing polyphenols, vitamin C, carotene and other biologically active components, fruits and honey are the supplements that can be useful in the management of As toxicity [217]. Ayurvedic therapies are also areas explored against As toxicity. However, the drug safety regulations are required [241,242]. Because As contamination have a big impact on human health, there are still challenges in the field of new treatment of the metal toxicity [243].
4. General discussion and comparison of the treatment between the five THM
The five THM are different in terms of application, toxicity and the target organs effects. However, there is similarity on their treatment. Following diagnosis of intoxication by the THM (Table 2), particularly in acute forms, general treatment usually starts by gastric aspiration and lavage and a saline laxative, soon after referral if clinically indicated. This might prevent further absorption of further amounts of the THM.
Table 2.
Clinical evaluating tools to diagnose and determine intoxication by THM.
| Medical history and physical examination | Ask about symptoms, occupational and other exposures, do a physical examination |
| Hematological and biochemical lab tests | Finding abnormal biochemical and hematological indices related to THM toxicity |
| THM blood and serum test | Measurement of THM level in whole blood or in serum |
| Complete blood count (CBC) | Finding abnormal blood indices related to THM toxicity |
| U/A | Urine evaluation for detection or quantification of THM |
| Hair, nail and saliva level of THM | Tissue evaluation for detection or quantification of THM |
| Electrocardiogram | ECG abnormalities may be observed hours to days after exposure |
| X-ray and CT scan imaging tests | Provide data of occurrence and location of abnormalities related to THM toxicity |
Some of the toxic metals require special attention. For example, brain edema management in lead poisoning, treatment of hemolytic reaction for arsenic and therapy for exudative dermatitis in chromium poisoning should be done. The use of chelating agents as THM antidotes is well known and research for the management of these THM poisonings using new chelators is ongoing. Along with the antidote therapy, particular attention on the target organs such as the CNS, kidneys and liver should be taken to reduce the organ damage. Fig. 1 briefly demonstrates the diagnose and clinical management of THM poisoning. Fig. 2 shows the schematic of chelation therapy for the clinical management of intoxication by the THM.
Fig. 1.
Exposure, diagnosis and clinical management of intoxication by THM; THM: toxic heavy metal.
Fig. 2.
Simple schematic expression of chelation therapy: Release of a toxic metal-bounded protein from THM by using chelating agents; THM: toxic heavy metal; R is used to represent the rest of the molecular structure of the chelating agent.
Chelating agents are the compounds which allow attachment of two or more donor atoms to the THM. This causes the formation of a stable complex in the form of a ring structure. Chelating agents are capable of effectively mobilizing THM deposits in the urine. Structure of suggested chelating agents used for the clinical management of the THM poisoning is shown in Fig. 3. There are potential risks and side effects associated with such treatments. For instance, chelating agents may cause renal toxicity and imbalances in electrolytes. One study identified renal adverse effects including proteinuria, increased serum creatinine, and the risk for Fanconi syndrome. The renal dysfunction was resolved upon discontinuation of the medicine [244]. Inadequate mobilization of THM metals from tissues represents another drawback of chelation therapy. Certain chelators, particularly hydrophilic ones like DMSA are effective in enhancing renal excretion but struggle to access and mobilize metals stored intracellularly. Consequently, this can result in incomplete detoxification of THM metals from the body [245].
Fig. 3.
Structure of suggested chelators for the clinical management of the THM poisoning.
Due to many drawbacks in the application of commonly used chelating agents, their administration in the clinical management of THM has come with limitations. Having sides effects which sometimes would be serious (Table 3), the loss of essential metals, redistribution of THM, inability to remove THM from intracellular compartments can be mentioned as such drawbacks. Constant endeavors to replace suggested known chelators with newer and less complicated ones or concomitant therapy of known chelating agents with newer compounds have attracted much more attention. Research aiming to find new chelating agents continued and has been reported during recent years. Recent studies indicate that the clinical management of poisoning by the THM with different severities has basically focused on the commonly available chelators by putting them into different regimen of therapies.
Table 3.
Suggested known chelators for the clinical management of the THM poisoning.
| Chelator | Mercury (Hg) | Lead (Pb) | Chromium (Cr) | Cadmium (Cd) | Arsenic (As) | Best Route of Administration | Side Effects/Considerations |
|---|---|---|---|---|---|---|---|
| Dimercaprol (BAL) | For acute inorganic Hg/contraindicated for organic Hg/limited efficacy in chronic poisoning | Suggested | Suggested | Cd-BAL complex is much more nephrotoxic than Cd itself | Limited efficacy in chronic poisoning | IM | Injection site pain, nausea, vomiting, fever, kidney toxicity, headache, restlessness, increased blood pressure, tachycardia |
| CaNa2EDTA | Suggested | Suggested | Non-optimal effectiveness | Non-optimal effectiveness | IV | Nausea, vomiting, fever, increased blood pressure, arthralgia, allergic reactions, local inflammation, nephrotoxicity, headache, anorexia, myalgia, fatigue, thirst, chills, cardiac complications | |
| D- penicillamine | For acute inorganic mercury | Significant adverse effects; currently replaced by succimer | Suggested | Ineffective or detrimental | Suggested | PO | Allergic reactions, immunodeficiency, worsening of neurological manifestations in patients with Wilson's disease |
| Succimer (DMSA) | For acute inorganic mercury | FDA-approved | Suggested | Suggested | Suggested: chelator of choice | PO | Nausea, vomiting, diarrhea, fever, hives, dizziness, weakness |
| Unithiol (DMPS) | For acute inorganic mercury | Suggested | Limited efficacy in chronic poisoning | PO, IV | Rash, nausea, leucopenia | ||
| DTPA | Suggested | IV |
IM: intramuscular injection, IV: intravenous injection, PO: oral administration.
Combination therapy aimed to use medications to act by different mechanism of actions. If the prescribed drugs act through a synergism action, if will enhance the efficacy of treatment. One might do combination therapy with known and newer chelators, while another will have the strategy of known chelating agent plus antioxidants [246]. Different treatment regimens were recommended mainly with application of natural thiol-containing substances which may have chelating properties for THM. Garlic is a natural compound containing thiol and has shown promising results in the treatment of lead poisoning. Some other investigations have used compounds with known antioxidant, anti-inflammatory, antiapoptotic and anti-cancer properties for the management of THM poisoning (Table 4). Such compounds include natural, synthetic or semi-synthetic substances that have undergone animal studies and the beneficial results of their usage were reported. Phenolic, flavonoid and tannin compounds which are enriched in fruits, show protective effects, are the sources of the reducing antioxidant power. On the other hand, experimental research is more directed towards finding novel treatment options, especially from botanical plants and herbal medicines. Some reports on animal studies provided important findings on the protective effects of the antioxidant and anti-inflammatory ingredients present in the natural products against THM poisoning [86,87,163,247]. In this regard, interest has been taken with reference to nano specifications of the compounds in order to gain higher therapeutic efficacy [70,240,248].
Table 4.
Frequently used supplements for the treatment of THM poisoning in recent literature.
| THM | Recently reported supplements |
|---|---|
| Hg | Shenkang injection, Calcium carbonate, garlic tablet and N-acetyl cysteine (NAC), Selenium supplementation = Selenomethionine and sodium selenite, α-LA, Flavonoids = Myricetin |
| Pb | D-penicillamine plus garlic, Moringa oleifera, ascorbic acid, silibinin and nano-silibinin, Silymarin, garlic, garlic combined with silymarin, Linum usitatissimum (Flaxseed) and Emblica Officinalis (Amla), lemon juice and Flaxseed, Ginkgo biloba, probiotic microorganisms [bacteria (Lactobacillus sp., Pediococcus pentosaceus, Bacillus sp.) and yeast (Saccharomyces cerevisiae)], micronutrients (such as zinc and vitamin C), herbal extracts (such as tea polyphenols and grape seed extract), Cinnamon and thymus vulgaris extract (thyme), Vit D, Glycine, NAC, TQ |
| Cr | NAC, ascorbic acid and NAC, vitamin C, Selenium supplementation, melatonin, Resveratrol, alpha-tocopherol, zinc |
| Cd | curcumin, ginger, resveratrol, physalis extract, grapefruit juice, allicin, royal jelly, spirulina, quercetin, caffeic acid phenethyl ester, vit C, vit E, NAC, coenzyme Q10, alpha-tocopherol, selenium and zinc, N-benzyl-D-glucamine dithiocarbamate (BGD), N-tetramethylene dithiocarbamate (ATC) and N-p-hydroxymethylbenzyl-D-glucamine dithiocarbamate (HBGD), Royal jelly, probiotic microorganisms, zinc, selenium, or iron |
| As | monoisoamyl 2,3-dimercaptosuccinic acid (monoisoamyl DMSA, MiADMSA), NAC, melatonin, green tea (Camellia sinensis), Malus domestica, Spirulina (Cyanobacteria), virgin coconut oil (VCO), ginger, Coriandrum sativum, Aspalathus linearis, Viscum album and Phyllanthus emblica leaves, Syzygium cumini leaves, Moringa oleifera leaves and Ipomea aquatica aerial parts, Flavonolignan (silibinin), Allium sativum, Curcuma longa, Silybum marianum, some herbal fibers and algae, vitamins A, C and E, zinc and selenium, folate, pyridoxine and riboflavin, β-carotene, α-tocopherol, genistein, α-LA, eriodictyol, hydroxytyrosol, epigallocatechin-3-gallate, oleuropein, ellagic acid, rutin, curcumin, quercetin, naringenin, sulforaphane, allicin, lutein, biochanin, resveratrol and D-pinitol, garlic, MiADMSA with curcumin, amino acids, flavonoids, polyphenols, jiggery, Ayurvedic therapies |
Table 3 presents the comparison of suggested known chelating agents that are commonly administered for the management of the five THM. Briefly, the following points were summarized:
-
I.
In order to prevent the loss of important nutritional metals such as calcium, iron, zinc during chelation therapies, monitoring of these elements in the urine are required.
-
II.
Adjusting the dose of chelators is a function of the serum level of the THM and the patient's symptoms and signs of toxicities.
-
III.
There is no specific antidote for Hg, but chelation therapy is favored in patients with moderate to severe intoxication.
-
IV.
Limited efficacy in chronic organic Hg poisoning has been shown for dimercaprol (BAL). BAL also redistributes this THM to the brain and is therefore contraindicated.
-
V.
Succimer has U.S. FDA approval for the clinical management of plumbism.
-
VI.
There are few evidences on the effect of known chelators in Cr intoxication. However, NAC and ascorbic acid have shown beneficial results either alone or in combination with the standard therapies.
-
VII.
Chelation therapy remains unproven for medical treatment of acute Cd poisoning. As for succimer, DMPS and DTPA are assumed to be effective chelating therapies.
-
VIII.
Cd-BAL complex has shown more nephrotoxic effects than Cd itself.
-
IX.
Nutritional combination therapy has shown more efficacious in clinical management of chronic As toxicity.
-
X.
There is no antidote for arsine gas poisoning. Clinical management is mainly supportive and no chelation therapy is warranted.
-
XI.
Chelation therapy has limited efficacy in chronic As toxicity.
-
XII.
Administration of dimercaprol should be done at least 4 h before the CaNa2EDTA in Pb poisoning.
-
XIII.
Although suggested for the management of Cr intoxication, EDTA has limited efficacy because of high removal of beneficial minerals such as zinc and calcium.
-
XIV.
Apart from lead poisoning, succimer may also be used in Hg or As toxicities.
Treatment of THM poisonings using different chelating agents may have the same healing results. For example, a treatment course of D-penicillamine plus garlic can have the same outcome as succimer does, but at a lower cost [74]. It seems that such chelator of choice is suitable when succimer is not available or the high cost will not be affordable.
Phytochemicals can provide protection against free radicals’ injury and help to maintain human health. For this reason, the use of phytochemicals together with conventional chelating agents has been considered. Curcumin, as an antioxidant and anti-inflammatory medication that has received much attention in recent years, has been shown to be effective in the removal of THM from the body [240]. Quercetin is commonly found in substantial quantities in plants, and possesses potent antioxidant properties. Quercetin can scavenge free radicals and chelate metals. The dietary antioxidant quercetin seems promising to attenuate the toxicity of environmental THM exposure. The catechol group in the B-ring may be responsible for metal chelation and in oxidation processes [249,250]. Quercetin administration during chelation therapy with MiADMSA shows beneficial effects on the protection of inhibited blood δ-aminolevulinic acid dehydratase (ALAD) activity and depletion of As level from target organs in mice [251]. Another animal study revealed that allicin and quercetin, alone or in combination, can improve the antioxidant potent of the liver and alleviate Pb-induced liver injury and apoptosis. The protection resulted through the PI3K signaling pathway and stronger effects achieved by their combination [252]. Similarly in a mice study on testicular germ cells, quercetin attenuated Cd-induced cell apoptosis by suppressing oxidative stress mediators and also via downregulation of the expression of Bax and caspase-3 and upregulation of Bcl-XL expression [253]. Some of these phytochemicals have not yet been tested in a randomized clinical trial. However, these medications could not be prescribed as a main treatment, but may be used as adjunct therapy.
5. Conclusion
Chelation therapy has been regarded as a promising medical intervention for THM poisonings. Its application for the management of THM poisoning is an important aspect of heavy metals detoxification that was reviewed. Succimer is a less toxic metal chelator in chelating agents’ family. It has the FDA approval for the clinical management of plumbism. Similarly, Dimercapto-propanesulfonic acid has also fewer side effects. The two are currently gaining increased acceptance among clinical toxicologists. However, there is no specific antidote for mercury poisoning. Dimercaprol is almost no longer being used as an antidote of choice in the treatment of chronic THM poisoning. Comparison of clinical management of the toxicity by the five heavy metals reveals similar treatment strategies to prevent more exposure, using chelating agents and to improve the antioxidant power. On the other hand, some of them require specific interventions to reduce the toxicity. Findings of many studies conducted in the recent years indicate that combination therapy using two chelating agents or co-administration of natural/synthetic antioxidants along with chelators is beneficial in getting more pronounced therapeutic efficacies.
Because of drawbacks in the application of commonly known chelating agents, treatment with bioactive compounds which have antioxidant and anti-inflammatory properties has been the subject of much interest in recent research. However, despite the promising results observed, clinical trials on their therapeutic benefits need to be established to determine the efficacy and safety in humans. Development of less toxic chelating agents has still very high research priority. Moreover, the development of orally administrable chelating agents for home health care would likely be of great interest for future research.
5.1. Limitations
Despite the recent advances in the development of clinical management of heavy metals intoxication, several limitations can be mentioned. Firstly, there is often a lack of comprehensive clinical trials that adequately assess the safety and efficacy of these novel therapies across diverse populations. Many studies are carried out in controlled settings, which may not accurately reflect real-world situations in which patients show different levels of exposure and underlying diseases. Additionally, the long-term effects and potential side effects of some treatments remain poorly understood, which requires further investigation. Variation in individual responses is another limitation to the treatments, which is affected by genetic, environmental, and health factors. Furthermore, this study deals with the treatment of poisoning of heavy metals in general and does not cover special human populations such as children, pregnant women, and individuals with underlying diseases. Lastly, while this review highlights several promising therapies, the accessibility and cost of these treatments in different regions may hold back their widespread implementation, especially in low-resource settings.
CRediT authorship contribution statement
Mahdi Balali-Mood: Writing – review & editing, Supervision, Methodology, Conceptualization. Nastaran Eizadi-Mood: Writing – original draft, Investigation, Formal analysis, Data curation. Hossein Hassanian Moghaddam: Writing – original draft, Investigation, Formal analysis, Data curation. Leila Etemad: Writing – original draft, Investigation, Formal analysis, Data curation. Mohammad Moshiri: Writing – original draft, Investigation, Formal analysis, Data curation. Maryam Vahabzadeh: Writing – original draft, Investigation, Formal analysis, Data curation. Mahmood Sadeghi: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Data availability
The data that support the findings of this study are outlined in the current review either as tables, figures, and context references.
Funding
The authors gained no financial support regarding the preparation of this manuscript.
Declaration of competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Glossary
- AKI
Acute kidney injury
- α-LA
alpha-lipoic acid
- ALAD
δ-aminolevulinic acid dehydratase
- APL:
Acute promyelocytic leukemia
- ARDS
Acute respiratory distress syndrome
- ATC
N-tetramethylene dithiocarbamate
- ATSDR
Agency for Toxic Substances and Disease Registry
- BAL:
British Anti-Lewisite
- BBB
Blood brain barrier
- BGD
N-benzyl-D-glucamine dithiocarbamate
- BLC
Blood lead concentration
- CaNa2EDTA
Calcium disodium ethylene diamine tetra acetic acid
- CdTe
Cd telluride
- CKD
Chronic kidney disease
- CNS
Central nervous system
- DMPS
2,3-dimercaptopropane sulfonate
- DMSA
Dimercaptosuccinic acid (succimer)
- DTPA
Diethylenetriaminepentaacetic acid
- ECG (EKG)
Electrocardiogram
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- GSH
Glutathione
- HBGD
N-p-hydroxymethylbenzyl-D-glucamine dithiocarbamate
- IARC
International Agency for Research on Cancer
- ICU
Intensive care unit
- MDA
Malondialdehyde
- MiADMSA
Monoisoamyl 2,3-dimercaptosuccinic acid
- MMP
Mitochondrial membrane potential
- NAC
N-acetylcysteine
- NIOSH
National Institute for Occupational Safety and Health
- OSHA
Occupational Safety and Health Administration
- PEL:
Permissible exposure limit
- PNS
Peripheral nervous system
- ROS
Reactive oxygen species
- VEGF
Vascular endothelial growth factor
- VCO
Virgin coconut oil
- WHO
World Health Organization
References
- 1.Balali-Mood M., Riahi-Zanjani B., Yousefzadeh H., Sadeghi M. Concentrations of mercury, lead, chromium, cadmium, arsenic and aluminum in irrigation water wells and wastewaters used for agriculture in Mashhad, northeastern Iran. Int. J. Occup. Environ. Med. 2013;4(2) [PubMed] [Google Scholar]
- 2.Mousavi S.R., Balali-Mood M., Riahi-Zanjani B., Sadeghi M. Determination of cyanide and nitrate concentrations in drinking, irrigation, and wastewaters. J. Res. Med. Sci.: the Official Journal of Isfahan University of Medical Sciences. 2013;18(1):65. [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati K., Scimeca J.-C., Ivanovski S., Verron E. Double-edged sword: therapeutic efficacy versus toxicity evaluations of doped titanium implants. Drug Discov. Today. 2021;26(11):2734–2742. doi: 10.1016/j.drudis.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Arredondo M., Núñez M.T. Iron and copper metabolism. Mol. Aspect. Med. 2005;26(4–5):313–327. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Farver O. Copper proteins. Protein electron transfer. Garland Science. 2020:161–188. [Google Scholar]
- 6.Ruiz L.M., Libedinsky A., Elorza A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.711227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallu R.K., Velugula K., Doshi S., Chinta J.P. Colorimetric and fluorimetric detection of Hg2+ and Cr3+ by boronic acid conjugated rhodamine derivatives: mechanistic aspects and their bio-imaging application in bacterial cells. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018;189:556–562. doi: 10.1016/j.saa.2017.08.052. [DOI] [PubMed] [Google Scholar]
- 8.Bao K., Wang X., Wang K., Yang Y., Li G. Effects of dietary supplementation with selenium and vitamin E on growth performance, nutrient apparent digestibility and blood parameters in female Sika deer (Cervus nippon) Biol. Trace Elem. Res. 2020;195(2):454–460. doi: 10.1007/s12011-019-01856-7. [DOI] [PubMed] [Google Scholar]
- 9.Vinceti M., Filippini T., Wise L.A. Environmental selenium and human health: an update. Current environmental health reports. 2018;5(4):464–485. doi: 10.1007/s40572-018-0213-0. [DOI] [PubMed] [Google Scholar]
- 10.Kianoush S., Sadeghi M., Balali-Mood M. Recent advances in the clinical management of lead poisoning. Acta Med. Iran. 2015:327–336. [PubMed] [Google Scholar]
- 11.Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacyna E., Pacyna J. Global emission of mercury from anthropogenic sources in 1995. Water Air Soil Pollut. 2002;137(1):149–165. [Google Scholar]
- 13.Wang Q., Kim D., Dionysiou D.D., Sorial G.A., Timberlake D. Sources and remediation for mercury contamination in aquatic systems—a literature review. Environmental pollution. 2004;131(2):323–336. doi: 10.1016/j.envpol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Rice K.M., Walker E.M., Jr., Wu M., Gillette C., Blough E.R. Environmental mercury and its toxic effects. Journal of preventive medicine and public health. 2014;47(2):74. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibau A.V., Grube B.D. Mercury contamination from dental amalgam. Journal of Health and Pollution. 2019;9(22) doi: 10.5696/2156-9614-9.22.190612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahaffey K. Proceedings of the 2004 National Forum on Contaminants in Fish. 2004. Update on recent epidemiologic mercury studies. [Google Scholar]
- 17.Driscoll C.T., Han Y.-J., Chen C.Y., Evers D.C., Lambert K.F., Holsen T.M., Kamman N.C., Munson R.K. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. Bioscience. 2007;57(1):17–28. [Google Scholar]
- 18.Sarikaya S., Karcioglu O., Ay D., Cetin A., Aktas C., Serinken M. Acute mercury poisoning: a case report. BMC Emerg. Med. 2010;10(1):1–3. doi: 10.1186/1471-227X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langford N., Ferner R. Toxicity of mercury. J. Hum. Hypertens. 1999;13(10):651–656. doi: 10.1038/sj.jhh.1000896. [DOI] [PubMed] [Google Scholar]
- 20.Fisher J.F., Organization W.H. World Health Organization; 2003. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. [Google Scholar]
- 21.Balluz L., Philen R., Sewell C., Voorhees R., Falter K., Paschal D. Mercury toxicity associated with a beauty lotion, New Mexico. Int. J. Epidemiol. 1997;26(5):1131–1132. doi: 10.1093/ije/26.5.1131. [DOI] [PubMed] [Google Scholar]
- 22.McRill C., Boyer L.V., Flood T.J., Ortega L. Mercury toxicity due to use of a cosmetic cream. Journal of occupational and environmental medicine. 2000:4–7. doi: 10.1097/00043764-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Control C.f.D., Prevention Mercury exposure among household users and nonusers of skin-lightning creams produced in Mexico-California and Virginia, 2010. MMWR. Morbidity and mortality weekly report. 2012;61(2):33–36. [PubMed] [Google Scholar]
- 24.Park J.-D., Zheng W. Human exposure and health effects of inorganic and elemental mercury. Journal of preventive medicine and public health. 2012;45(6):344. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copan L., Fowles J., Barreau T., McGee N. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel) Int. J. Environ. Res. Publ. Health. 2015;12(9):10943–10954. doi: 10.3390/ijerph120910943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramli F.F. Clinical management of chronic mercury intoxication secondary to skin lightning products: a proposed algorithm. Bosn. J. Basic Med. Sci. 2021;21(3):261. doi: 10.17305/bjbms.2020.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T., Shimohata T. Vascular dysfunction induced by mercury exposure. Int. J. Mol. Sci. 2019;20(10):2435. doi: 10.3390/ijms20102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira F.B., de Oliveira A.C.A., Leão L.K.R., Fagundes N.C.F., Fernandes R.M., Fernandes L.M.P., da Silva M.C.F., Amado L.L., Sagica F.E.S., de Oliveira E.H.C., Crespo-Lopez M.E., Maia C.S.F., Lima R.R. Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front. Mol. Neurosci. 2018;11 doi: 10.3389/fnmol.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han B., Lv Z., Han X., Li S., Han B., Yang Q., Wang X., Wu P., Li J., Deng N., Zhang Z. Harmful effects of inorganic mercury exposure on kidney cells: mitochondrial dynamics disorder and excessive oxidative stress. Biol. Trace Elem. Res. 2022;200(4):1591–1597. doi: 10.1007/s12011-021-02766-3. [DOI] [PubMed] [Google Scholar]
- 30.Broussard L.A., Hammett-Stabler C.A., Winecker R.E., Ropero-Miller J.D. The toxicology of mercury. Laboratory medicine. 2002;33(8):614–625. [Google Scholar]
- 31.Clarkson T. "The three modern faces of mercury. Environ. Health Perspect. 2002;110(suppl 1):11–23. doi: 10.1289/ehp.02110s111. Find this article online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafati-Rahimzadeh M., Rafati-Rahimzadeh M., Kazemi S., Moghadamnia A.A. Current approaches of the management of mercury poisoning: need of the hour. Daru. 2014;22(1):1–10. doi: 10.1186/2008-2231-22-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sofia H., Sugiharto E. Acute and chronic toxicity of mercury exposure in seafood and human populations near a small-scale gold mining area. Int J Public Health Sci. 2016;5:257–266. [Google Scholar]
- 34.Posin S.L., Kong E.L., Sharma S. Mercury toxicity. In: StatPearls. StatPearls Publishing. Treasure Island (FL) 2023;PMID:29763110. [PubMed] [Google Scholar]
- 35.Cheuk A., Chan H. Minimal change disease following exposure to mercury-containing skin lightning cream. Hong Kong Med. J. 2006;12(4):316–318. [PubMed] [Google Scholar]
- 36.Tang H.-L., Mak Y.-F., Chu K.-H., Lee W., Fung S., Chan T., Tong K.-L. Minimal change disease caused by exposure to mercury-containing skin lightning cream: a report of 4 cases. Clin. Nephrol. 2013;79(4):326–329. [PubMed] [Google Scholar]
- 37.Zhang L., Liu F., Peng Y., Sun L., Chen C. Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightning cream. Annals of Saudi medicine. 2014;34(3):257–261. doi: 10.5144/0256-4947.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Z., Wu N., Du X., Li H., Mei X., Song Y. Toxic nephropathy secondary to chronic mercury poisoning: clinical characteristics and outcomes. Kidney International Reports. 2022;7(6):1189–1197. doi: 10.1016/j.ekir.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye B.-J., Kim B.-G., Jeon M.-J., Kim S.-Y., Kim H.-C., Jang T.-W., Chae H.-J., Choi W.-J., Ha M.-N., Hong Y.-S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Annals of Occupational and Environmental Medicine. 2016;28(1):1–8. doi: 10.1186/s40557-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanusa M., Varnai V.M., Piasek M., Kostial K. Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr. Med. Chem. 2005;12(23):2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 41.Aaseth J., Ajsuvakova O.P., Skalny A.V., Skalnaya M.G., Tinkov A.A. Chelator combination as therapeutic strategy in mercury and lead poisonings. Coord. Chem. Rev. 2018;358:1–12. [Google Scholar]
- 42.Yawei S., Jianhai L., Junxiu Z., Xiaobo P., Zewu Q. Epidemiology, clinical presentation, treatment, and follow‐up of chronic mercury poisoning in China: a retrospective analysis. BMC Pharmacology and Toxicology. 2021;22(1):1–9. doi: 10.1186/s40360-021-00493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falluel‐Morel A., Lin L., Sokolowski K., McCandlish E., Buckley B., DiCicco‐Bloom E. N‐acetyl cysteine treatment reduces mercury‐induced neurotoxicity in the developing rat hippocampus. J. Neurosci. Res. 2012;90(4):743–750. doi: 10.1002/jnr.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwokocha C.R., Owu D.U., Nwokocha M.I., Ufearo C.S., Iwuala M.O. Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of wistar rats. Food Chem. Toxicol. 2012;50(2):222–226. doi: 10.1016/j.fct.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Vahabzadeh M., Balali-Mood M. Occupational metallic mercury poisoning in gilders. Int. J. Occup. Environ. Med. 2016;7(2):116. doi: 10.15171/ijoem.2016.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan M.A., Wang F. Mercury‐selenium compounds and their toxicological significance: toward a molecular understanding of the mercury‐selenium antagonism. Environ. Toxicol. Chem.: Int. J. 2009;28(8):1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- 47.Spiller H.A., Hays H.L., Casavant M.J. Rethinking treatment of mercury poisoning: the roles of selenium, acetylcysteine, and thiol chelators in the treatment of mercury poisoning: a narrative review. Toxicology Communications. 2021;5(1):19–59. [Google Scholar]
- 48.Hasanein P., Emamjomeh A. Dietary Interventions in Liver Disease. Elsevier; 2019. Beneficial effects of natural compounds on heavy metal–induced hepatotoxicity; pp. 345–355. [Google Scholar]
- 49.Makena W., Aribiyun Y.S., Aminu A., Ishaku B., Yohana A., Inemesit E.E. Flavonoids fractions of Adansonia digitata L. fruits protects adult Wistar rats from mercury chloride-induced hepatorenal toxicity: histopathological and biochemical studies. Egyptian Journal of Basic and Applied Sciences. 2022;9(1):205–215. [Google Scholar]
- 50.Franco J.L., Posser T., Missau F., Pizzolatti M.G., Santos A.R., Souza D.O., Aschner M., Rocha J.B., Dafre A.L., Farina M. Structure–activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ. Toxicol. Pharmacol. 2010;30(3):272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vahabzadeh M., Mégarbane B. Abdominal pain related to adulterated opium: an emerging issue in drug addicts. World J. Psychiatr. 2020;10(5):95. doi: 10.5498/wjp.v10.i5.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.OSHA . 2020. "Toxic and Hazardous Substances: Lead. [Google Scholar]
- 53.CDC . 2022. "Center for Disease Control and Prevention: Blood Lead Levels in Children ". [Google Scholar]
- 54.Liu B., Zhang H., Tan X., Yang D., Lv Z., Jiang H., Lu J., Baiyun R., Zhang Z. GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget. 2017;8(26) doi: 10.18632/oncotarget.15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chibowska K., Baranowska-Bosiacka I., Falkowska A., Gutowska I., Goschorska M., Chlubek D. Effect of lead (Pb) on inflammatory processes in the brain. Int. J. Mol. Sci. 2016;17(12):2140. doi: 10.3390/ijms17122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh N., Kumar A., Gupta V.K., Sharma B. Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem. Res. Toxicol. 2018;31(10):1009–1021. doi: 10.1021/acs.chemrestox.8b00193. [DOI] [PubMed] [Google Scholar]
- 57.Metryka E., Chibowska K., Gutowska I., Falkowska A., Kupnicka P., Barczak K., Chlubek D., Baranowska-Bosiacka I. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018;19(6):1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce J. Burton's line in lead poisoning. Eur. Neurol. 2007;57(2):118–119. doi: 10.1159/000098100. [DOI] [PubMed] [Google Scholar]
- 59.Samarghandian S., Shirazi F.M., Saeedi F., Roshanravan B., Pourbagher-Shahri A.M., Khorasani E.Y., Farkhondeh T., Aaseth J.O., Abdollahi M., Mehrpour O. A systematic review of clinical and laboratory findings of lead poisoning: lessons from case reports. Toxicol. Appl. Pharmacol. 2021;429 doi: 10.1016/j.taap.2021.115681. [DOI] [PubMed] [Google Scholar]
- 60.Etemadi A., Hariri S., Hassanian-Moghaddam H., Poustchi H., Roshandel G., Shayanrad A., Kamangar F., Boffetta P., Brennan P., Dargan P.I. Lead poisoning among asymptomatic individuals with a long-term history of opiate use in Golestan Cohort Study. Int. J. Drug Pol. 2022;104 doi: 10.1016/j.drugpo.2022.103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Organization W.H. World Health Organization; 2021. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. [PubMed] [Google Scholar]
- 62.Ekinci M., Ceylan E., Çağatay H.H., Keleş S., Altınkaynak H., Kartal B., Koban Y., Hüseyinoğlu N. Occupational exposure to lead decreases macular, choroidal, and retinal nerve fiber layer thickness in industrial battery workers. Curr. Eye Res. 2014;39(8):853–858. doi: 10.3109/02713683.2013.877934. [DOI] [PubMed] [Google Scholar]
- 63.Vahabzadeh M., Balali-Mood M., Banagozar Mohammadi A., Moshiri M. Efficacy and expenses of succimer vs. d-penicillamine plus garlic in the treatment of lead poisoning: a retrospective cross-sectional study. Daru. 2021;29(2):477–481. doi: 10.1007/s40199-021-00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Committee on Environmental Health Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116(4):1036–1046. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- 65.Payne M. Lead in drinking water. CMAJ (Can. Med. Assoc. J.) 2008;179(3):253–254. doi: 10.1503/cmaj.071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paeezi M., Zamani N., Hassanian-Moghaddam H., Shadnia S., Zamani N., Chaleshi V., Mafi A.A. Treatment of adult lead poisoning with D-penicillamine. Drug Metabolism and Personalized Therapy. 2019;34(2) doi: 10.1515/dmpt-2019-0003. [DOI] [PubMed] [Google Scholar]
- 67.Bhattacharya S. Probiotics against alleviation of lead toxicity: recent advances. Interdiscip. Toxicol. 2019;12(2):89. doi: 10.2478/intox-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Boshy M.E., Refaat B., Qasem A.H., Khan A., Ghaith M., Almasmoum H., Mahbub A., Almaimani R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Control Ser. 2019;26(22):22736–22746. doi: 10.1007/s11356-019-05562-8. [DOI] [PubMed] [Google Scholar]
- 69.Albasher G., Al Kahtani S., Alwahibi M.S., Almeer R. Effect of Moringa oleifera Lam. methanolic extract on lead-induced oxidative stress-mediated hepatic damage and inflammation in rats. Environ. Sci. Pollut. Control Ser. 2020;27(16):19877–19887. doi: 10.1007/s11356-020-08525-6. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadi M., Ariafar S., Talebi-Ghane E., Afzali S. Comparative efficacy of silibinin and nano-silibinin on lead poisoning in Male Wistar rats. Toxicology. 2022;475 doi: 10.1016/j.tox.2022.153242. [DOI] [PubMed] [Google Scholar]
- 71.Usman A., Kawu M.U., Shittu M., Saleh A., Jolayemi K.O., Ibrahim N.B., Oyetunde J.S., Okoronkwo M.O. Comparative effects of methanol leaf extract of Moringa oleifera and ascorbic acid on haematological and histopathological changes induced by subchronic lead toxicity in male wistar rats. Pharmacological Research-Modern Chinese Medicine. 2022;2 [Google Scholar]
- 72.Molavi N., Meamar R., Hasanzadeh A., Eizadi-Mood N. The effects of succimer and penicillamine on acute lead poisoning patients. Int. J. Med. Toxicol. Forensic Med. 2021;11(3) 33474-33474. [Google Scholar]
- 73.Jalali S.M., Najafzadeh H., Bahmei S. Protective role of silymarin and D-penicillamine against lead-induced liver toxicity and oxidative stress. Toxicol. Ind. Health. 2017;33(6):512–518. doi: 10.1177/0748233716685660. [DOI] [PubMed] [Google Scholar]
- 74.Kianoush S., Balali‐Mood M., Mousavi S.R., Moradi V., Sadeghi M., Dadpour B., Rajabi O., Shakeri M.T. Comparison of therapeutic effects of garlic and d‐Penicillamine in patients with chronic occupational lead poisoning. Basic Clin. Pharmacol. Toxicol. 2012;110(5):476–481. doi: 10.1111/j.1742-7843.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 75.Parsi A., Ghorbani A., Hesam S., Hosseini M. Comparison of Garlic therapeutic effects and standard therapy with De Penicillamine in patients with Lead poisoning. J. Adv. Pharm. Educ. Res. 2020;10(S2):85. [Google Scholar]
- 76.El-Khishin I.A., El-Fakharany Y.M.M., Hamid O.I.A. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol. Rep. 2015;2:824–832. doi: 10.1016/j.toxrep.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paul A., Sujatha K., Srilatha C., Kumar N.V. 2022. "Effect of Lead Toxicity on Liver and Kidney in Wistar Rats and its Amelioration with Linum usitatissimum (Flaxseed) and Emblica Officinalis (amla).". [Google Scholar]
- 78.Mohamed R.S., Fouda K., Akl E.M. Hepatorenal protective effect of flaxseed protein isolate incorporated in lemon juice against lead toxicity in rats. Toxicol. Rep. 2020;7:30–35. doi: 10.1016/j.toxrep.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asiwe J.N., Daubry T.M.E., Okon I.A., Akpotu A.E., Adagbada E.O., Eruotor H., Agbugba L.C., Buduburisi B.R. Ginkgo biloba supplement reverses lead (II) acetate–induced haematological imbalances, hepatic and renal dysfunctions in male wistar rat. Biol. Trace Elem. Res. 2022:1–11. doi: 10.1007/s12011-022-03098-6. [DOI] [PubMed] [Google Scholar]
- 80.Hamdan M., As N., Rahayu M., Machfoed M.H. Influence of ginkgo biloba (Egb) extracts in apoptosis index and number of neurons at Rattus novergicus with lead (pb) exposure. Res. J. Pharm. Technol. 2019;12(12):5883–5887. [Google Scholar]
- 81.Adebayo O.G., Ben-Azu B., Ajayi A.M., Wopara I., Aduema W., Kolawole T.A., Umoren E.B., Onyeleonu I., Ebo O.T., Ajibo D.N. Gingko biloba abrogate lead-induced neurodegeneration in mice hippocampus: involvement of NF-κB expression, myeloperoxidase activity and pro-inflammatory mediators. Biol. Trace Elem. Res. 2022;200(4):1736–1749. doi: 10.1007/s12011-021-02790-3. [DOI] [PubMed] [Google Scholar]
- 82.Hassan E., El-Neweshy M., Hassan M., Noreldin A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: possible mechanisms are involved. Life Sci. 2019;230:132–140. doi: 10.1016/j.lfs.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 83.Ata S.A., Abu-Dari K.I., Tutunji M.F., Mubarak M.S. Reversing the adverse biochemical effects in lead-intoxicated rats by N, N-bis [(1, 2-didehydro-1-hydroxy-2-thioxopyrid-4-yl)-carbonyl]-L-lysine. J. Trace Elem. Med. Biol. 2018;50:93–99. doi: 10.1016/j.jtemb.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Zhai Q., Yang L., Zhao J., Zhang H., Tian F., Chen W. Protective effects of dietary supplements containing probiotics, micronutrients, and plant extracts against lead toxicity in mice. Front. Microbiol. 2018;9:2134. doi: 10.3389/fmicb.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamed H.S., Ismal S.M., Abdel-Tawwab M. Modulatory effects of dietary cinnamon (Cinnamomum zeylanicum) against waterborne lead toxicity in Nile tilapia fingerlings: growth performance, haemato-biochemical, innate immunity, and hepatic antioxidant indices. Aquaculture Reports. 2022;25 [Google Scholar]
- 86.BaSalamah M.A., Abdelghany A.H., El-Boshy M., Ahmad J., Idris S., Refaat B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-23258-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ommati M.M., Ahmadi H.N., Sabouri S., Retana‐Marquez S., Abdoli N., Rashno S., Niknahad H., Jamshidzadeh A., Mousavi K., Rezaei M. Glycine protects the male reproductive system against lead toxicity via alleviating oxidative stress, preventing sperm mitochondrial impairment, improving kinematics of sperm, and blunting the downregulation of enzymes involved in the steroidogenesis. Environ. Toxicol. 2022;37(12):2990–3006. doi: 10.1002/tox.23654. [DOI] [PubMed] [Google Scholar]
- 88.Abedini Bajgiran F., Khazaei Koohpar Z., Salehzadeh A. Effects of N-acetylcysteine supplementation on oxidative stress and expression of apoptosis-related genes in testicular tissue of rats exposed to lead. Biol. Trace Elem. Res. 2022:1–9. doi: 10.1007/s12011-022-03325-0. [DOI] [PubMed] [Google Scholar]
- 89.Dayan A., Paine A. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001;20(9):439–451. doi: 10.1191/096032701682693062. [DOI] [PubMed] [Google Scholar]
- 90.Costa M., Klein C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006;36(2):155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- 91.Chakraborty R., Renu K., Eladl M.A., El-Sherbiny M., Elsherbini D.M.A., Mirza A.K., Vellingiri B., Iyer M., Dey A., Gopalakrishnan A.V. Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113119. [DOI] [PubMed] [Google Scholar]
- 92.Cheremisinoff N. John Wiley & Sons, Inc.; Hoboken, NJ: 2016. Agency for Toxic Substances and Disease Registry (ATSDR) p. 83. [Google Scholar]
- 93.Ventura C., Gomes B.C., Oberemm A., Louro H., Huuskonen P., Mustieles V., Fernández M.F., Ndaw S., Mengelers M., Luijten M. Biomarkers of effect as determined in human biomonitoring studies on hexavalent chromium and cadmium in the period 2008–2020. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.110998. [DOI] [PubMed] [Google Scholar]
- 94.Xu J., Zhao M., Pei L., Zhang R., Liu X., Wei L., Yang M., Xu Q. Oxidative stress and DNA damage in a long-term hexavalent chromium-exposed population in North China: a cross-sectional study. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2017-021470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li P., Li Y., Zhang J., Yu S.F., Tong W., Hu X., Jia G. Biomarkers for lung epithelium injury in occupational hexavalent chromium-exposed workers. Journal of occupational and environmental medicine. 2015;57(4):e45–e50. doi: 10.1097/JOM.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 96.Singhal V.K., Deswal B.S., Singh B.N. Study of skin and mucous membrane disorders among workers engaged in the sodium dichromate manufacturing industry and chrome plating industry. Indian J. Occup. Environ. Med. 2015;19(3):129. doi: 10.4103/0019-5278.173994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aminian O., Eftekhari S., Mazaheri M., Sharifian S.A., Sadeghniiat-Haghighi K. Urinary β2 microglobulin in workers exposed to arc welding fumes. Acta Med. Iran. 2011:748–752. [PubMed] [Google Scholar]
- 98.Yatera K., Morimoto Y., Ueno S., Noguchi S., Kawaguchi T., Tanaka F., Suzuki H., Higashi T. Cancer risks of hexavalent chromium in the respiratory tract. J. UOEH. 2018;40(2):157–172. doi: 10.7888/juoeh.40.157. [DOI] [PubMed] [Google Scholar]
- 99.Proctor D.M., Otani J.M., Finley B.L., Paustenbach D.J., Bland J.A., Speizer N., Sargent E.V. Is hexavalent chromium carcinogenic via ingestion? A weight-of-evidence review. J. Toxicol. Environ. Health, Part A. 2002;65(10):701–746. doi: 10.1080/00984100290071018. [DOI] [PubMed] [Google Scholar]
- 100.Gatto N.M., Kelsh M.A., Mai D.H., Suh M., Proctor D.M. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer epidemiology. 2010;34(4):388–399. doi: 10.1016/j.canep.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 101.Welling R., Beaumont J.J., Petersen S.J., Alexeeff G.V., Steinmaus C. Chromium VI and stomach cancer: a meta-analysis of the current epidemiological evidence. Occup. Environ. Med. 2015;72(2):151–159. doi: 10.1136/oemed-2014-102178. [DOI] [PubMed] [Google Scholar]
- 102.Shelnutt S.R., Goad P., Belsito D.V. Dermatological toxicity of hexavalent chromium. Crit. Rev. Toxicol. 2007;37(5):375–387. doi: 10.1080/10408440701266582. [DOI] [PubMed] [Google Scholar]
- 103.Cerulli J., Grabe D.W., Gauthier I., Malone M., McGoldrick M.D. Chromium picolinate toxicity. Ann. Pharmacother. 1998;32(4):428–431. doi: 10.1345/aph.17327. [DOI] [PubMed] [Google Scholar]
- 104.Rall D.P., Pope A.M. 1995. "Environmental Medicine: Integrating a Missing Element into Medical education.". [PubMed] [Google Scholar]
- 105.Stift A., Friedl J., Lñgle F., Berlakovich G., Steininger R., Mühlbacher F. Successful treatment of a patient suffering from severe acute potassium dichromate poisoning with liver transplantation. Transplantation. 2000;69(11):2454–2455. doi: 10.1097/00007890-200006150-00044. [DOI] [PubMed] [Google Scholar]
- 106.Sun H., Brocato J., Costa M. Oral chromium exposure and toxicity. Current environmental health reports. 2015;2(3):295–303. doi: 10.1007/s40572-015-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ellis E.N., Brouhard B.H., Lynch R.E., Dawson E.B., Tisdell R., Nichols M.M., Ramirez F. Effects of hemodialysis and dimercaprol in acute dichromate poisoning. J. Toxicol. Clin. Toxicol. 1982;19(3):249–258. doi: 10.3109/15563658209025729. [DOI] [PubMed] [Google Scholar]
- 108.Schiffl H., Weidmann P., Weiss M., Massry S.G. Dialysis treatment of acute chromium intoxication and comparative efficacy of peritoneal versus hemodialysis in chromium removal. Miner. Electrolyte Metab. 1982;7(1):28–35. [PubMed] [Google Scholar]
- 109.Smith S.W. The role of chelation in the treatment of other metal poisonings. J. Med. Toxicol. 2013;9(4):355–369. doi: 10.1007/s13181-013-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pazzaglia U., Apostoli P., Congiu T., Catalani S., Marchese M., Zarattini G. Cobalt, chromium and molybdenum ions kinetics in the human body: data gained from a total hip replacement with massive third body wear of the head and neuropathy by cobalt intoxication. Arch. Orthop. Trauma Surg. 2011;131(9):1299–1308. doi: 10.1007/s00402-011-1268-7. [DOI] [PubMed] [Google Scholar]
- 111.Poonam N.T., Devvret K.P., Anju Rani M.P. Detoxification of arsenic and chromium through chelators and enzymes: an in-silico approach. Int. J. Appl. Eng. Res. 2018;13(10):8249–8271. [Google Scholar]
- 112.Anderson R.A., Bryden N.A., Waters R. EDTA chelation therapy does not selectively increase chromium losses. Biol. Trace Elem. Res. 1999;70(3):265–272. doi: 10.1007/BF02783835. [DOI] [PubMed] [Google Scholar]
- 113.Molina-Jijón E., Zarco-Márquez G., Medina-Campos O.N., Zataraín-Barrón Z.L., Hernández-Pando R., Pinzón E., Zavaleta R.M., Tapia E., Pedraza-Chaverri J. Deferoxamine pretreatment prevents Cr (VI)-induced nephrotoxicity and oxidant stress: role of Cr (VI) chelation. Toxicology. 2012;291(1–3):93–101. doi: 10.1016/j.tox.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Iranmanesh M., Fatemi S.J.A., Ebrahimpour R., Dahooee Balooch F. Chelation of chromium (VI) by combining deferasirox and deferiprone in rats. Biometals. 2013;26(3):465–471. doi: 10.1007/s10534-013-9631-5. [DOI] [PubMed] [Google Scholar]
- 115.Nabavi N., Moshiri M., Tajoddini S., Dadpour B. A basis for the decision to rule in or out acetaminophen toxicity: assessment of the serum level within 4 hours post overdose. Iranian Journal of Toxicology. 2021;15(4):265–270. [Google Scholar]
- 116.Jaafarzadeh M., Mahjoob Khaligh R., Mohsenifar Z., Shabani A., Rezvani Gilkalaei M., Rajabi Keleshteri S., Beigi Harchegani A. Protecting effects of N-acetyl cysteine supplementation against lead and cadmium-induced brain toxicity in rat models. Biol. Trace Elem. Res. 2022;200(10):4395–4403. doi: 10.1007/s12011-021-03034-0. [DOI] [PubMed] [Google Scholar]
- 117.Hena Z., McCabe M.E., Perez M.M., Sharma M., Sutton N.J., Peek G.J., Clark B.C. Aluminum phosphide poisoning: successful recovery of multiorgan failure in a pediatric patient. International journal of pediatrics and adolescent medicine. 2018;5(4):155–158. doi: 10.1016/j.ijpam.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yeh S.T.-Y., Guo H.-R., Su Y.-S., Lin H.-J., Hou C.-C., Chen H.-M., Chang M.-C., Wang Y.-J. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223(3):181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 119.Rossignol D.A. The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Springer; 2019. The use of N-acetylcysteine as a chelator for metal toxicity; pp. 169–179. [Google Scholar]
- 120.Boşgelmez İ.İ. An overview on the potential mechanisms of action of N-acetyl-l-cysteine in hexavalent chromium-induced toxicity. Toxicology. 2021:397–408. [Google Scholar]
- 121.Luczak M.W., Zhitkovich A. Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium (VI), cadmium (II), and cobalt (II) Free Radic. Biol. Med. 2013;65:262–269. doi: 10.1016/j.freeradbiomed.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee Y.-H., Su S.-B., Huang C.-C., Sheu H.-M., Tsai J.-C., Lin C.-H., Wang Y.-J., Wang B.-J. N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang B.J., Guo Y.L., Chang H.Y., Sheu H.M., Pan M.H., Lee Y.H., Wang Y.J. N‐acetylcysteine inhibits chromium hypersensitivity in coadjuvant chromium‐sensitized albino Guinea pigs by suppressing the effects of reactive oxygen species. Experimental dermatology. 2010;19(8):e191–e200. doi: 10.1111/j.1600-0625.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 124.Ahmed M.A.E.-S., Ashraf M.A.-M., Hala M.A.-K. N-acetylcysteine an Allium plant compound protects against chromium (VI) induced oxidant stress and ultrastructural changes of pancreatic beta-cells in rats. J. Med. Plants Res. 2010;4(21):2290–2297. [Google Scholar]
- 125.Yu J.-H., Lu J.-X., Smollin C., Cheng H.-T., Seak C.-J., Chen H.-Y. N-acetylcysteine and ascorbic acid therapy for acute hepatic injury after hexavalent chromium ingestion. J. Clin. Pharm. Therapeut. 2019;45(1):208–210. doi: 10.1111/jcpt.13044. [DOI] [PubMed] [Google Scholar]
- 126.Giampreti A., Lonati D., Ragghianti B., Ronchi A., Petrolini V.M., Vecchio S., Locatelli C.A., N-acetyl-cysteine as effective and safe chelating agent in metal-on-metal hip-implanted patients: two cases, Case reports in orthopedics 2016 (2016) (1):8682737. [DOI] [PMC free article] [PubMed]
- 127.Lin C.-C., Wu M.-L., Yang C.-C., Ger J., Tsai W.-J., Deng J.-F. Acute severe chromium poisoning after dermal exposure to hexavalent chromium. J. Chin. Med. Assoc. 2009;72(4):219–221. doi: 10.1016/S1726-4901(09)70059-0. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., Liu Y., Wan H., Zhu Y., Chen P., Hao P., Cheng Z., Liu J. Moderate selenium dosing inhibited chromium (VI) toxicity in chicken liver. J. Biochem. Mol. Toxicol. 2017;31(8) doi: 10.1002/jbt.21916. [DOI] [PubMed] [Google Scholar]
- 129.Banerjee S., Joshi N., Mukherjee R., Singh P.K., Baxi D., Ramachandran A. Melatonin protects against chromium (VI) induced hepatic oxidative stress and toxicity: duration dependent study with realistic dosage. Interdiscip. Toxicol. 2017;10(1):20. doi: 10.1515/intox-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Banu S.K., Stanley J.A., Sivakumar K.K., Arosh J.A., Burghardt R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016;303:65–78. doi: 10.1016/j.taap.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dey S.K., Nayak P., Roy S. Alpha-tocopherol supplementation on chromium toxicity: a study on rat liver and kidney cell membrane. Journal of Environmental Sciences. 2003;15(3):356–359. [PubMed] [Google Scholar]
- 132.Wang M., Chen Z., Song W., Hong D., Huang L., Li Y. A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull. Environ. Contam. Toxicol. 2021;106(1):65–74. doi: 10.1007/s00128-020-03088-1. [DOI] [PubMed] [Google Scholar]
- 133.Pinot F., Kreps S.E., Bachelet M., Hainaut P., Bakonyi M., Polla B.S. Cadmium in the environment: sources, mechanisms of biotoxicity, and biomarkers. Rev. Environ. Health. 2000;15(3):299–324. doi: 10.1515/reveh.2000.15.3.299. [DOI] [PubMed] [Google Scholar]
- 134.Haider F.U., Liqun C., Coulter J.A., Cheema S.A., Wu J., Zhang R., Wenjun M., Farooq M. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2020.111887. [DOI] [PubMed] [Google Scholar]
- 135.Ganguly K., Levänen B., Palmberg L., Åkesson A., Lindén A. Cadmium in tobacco smokers: a neglected link to lung disease? Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0122-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang H., Reynolds M. Cadmium exposure in living organisms: a short review. Sci. Total Environ. 2019;678:761–767. doi: 10.1016/j.scitotenv.2019.04.395. [DOI] [PubMed] [Google Scholar]
- 137.Schaefer H.R., Dennis S., Fitzpatrick S. Cadmium: mitigation strategies to reduce dietary exposure. Journal of food science. 2020;85(2):260–267. doi: 10.1111/1750-3841.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matt G.E., Quintana P.J., Hoh E., Dodder N.G., Mahabee-Gittens E.M., Padilla S., Markman L., Watanabe K. Tobacco smoke is a likely source of lead and cadmium in settled house dust. J. Trace Elem. Med. Biol. 2021;63 doi: 10.1016/j.jtemb.2020.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arini A., Feurtet-Mazel A., Maury-Brachet R., Pokrovsky O.S., Coste M. Recovery potential of periphytic biofilms translocated in artificial streams after industrial contamination (Cd and Zn) Ecotoxicology. 2012;21(5):1403–1414. doi: 10.1007/s10646-012-0894-3. [DOI] [PubMed] [Google Scholar]
- 140.Nand V., Maata M., Koshy K., Sotheeswaran S. Water purification using moringa oleifera and other locally available seeds in Fiji for heavy metal removal. International Journal of Applied. 2012;2(5):125–129. [Google Scholar]
- 141.Hu J., Li M., Han T.-x., Chen J.-w., Ye L.-x., Wang Q., Zhou Y.-k. Benchmark dose estimation for cadmium-induced renal tubular damage among environmental cadmium-exposed women aged 35–54 years in two counties of China. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wallin M., Sallsten G., Lundh T., Barregard L. Low-level cadmium exposure and effects on kidney function. Occup. Environ. Med. 2014;71(12):848–854. doi: 10.1136/oemed-2014-102279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rahimzadeh M.R., Rahimzadeh M.R., Kazemi S., Moghadamnia A.-a. Cadmium toxicity and treatment: an update. Caspian journal of internal medicine. 2017;8(3):135. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The effects of cadmium toxicity. Int. J. Environ. Res. Publ. Health. 2020;17(11):3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang B., Du Y. Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lorentzon E., Horvath I., Kumar R., Rodrigues J.I., Tamás M.J., Wittung-Stafshede P. Effects of the toxic metals arsenite and cadmium on α-synuclein aggregation in vitro and in cells. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tellez-Plaza M., Jones M.R., Dominguez-Lucas A., Guallar E., Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atheroscler Rep. 2013;15(10):1–15. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y., Zhang Y., Feng R., Zheng P., Huang H., Zhou S., Ji W., Huang F., Liu H., Zhang G. Cadmium induces testosterone synthesis disorder by testicular cell damage via TLR4/MAPK/NF-κB signaling pathway leading to reduced sexual behavior in piglets. Ecotoxicol. Environ. Saf. 2022;233 doi: 10.1016/j.ecoenv.2022.113345. [DOI] [PubMed] [Google Scholar]
- 149.Cancer I.A.f.R.o. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012. Arsenic, metals, fibres and dusts. [PMC free article] [PubMed] [Google Scholar]
- 150.Luevano J., Damodaran C. "A review of molecular events of cadmium-induced carcinogenesis." Journal of Environmental Pathology. Toxicology and Oncology. 2014;33(3) doi: 10.1615/jenvironpatholtoxicoloncol.2014011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park E., Kim S., Song S.-H., Lee C.-W., Kwon J.-T., Lim M.K., Park E.Y., Won Y.-J., Jung K.-W., Kim B. Environmental exposure to cadmium and risk of thyroid cancer from national industrial complex areas: a population-based cohort study. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.128819. [DOI] [PubMed] [Google Scholar]
- 152.Landrigan P.J., Lucchini R.G., Kotelchuck D., Grandjean P. Handbook on the Toxicology of Metals. Elsevier; 2022. Principles for prevention of the toxic effects of metals; pp. 685–703. [Google Scholar]
- 153.Malejko J., Deoniziak K., Tomczuk M., Długokencka J., Godlewska-Żyłkiewicz B. Puparial cases as toxicological indicators: bioaccumulation of cadmium and thallium in the forensically important blowfly Lucilia sericata. Frontiers in chemistry. 2020;8 doi: 10.3389/fchem.2020.586067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gonick H. Nephrotoxicity of cadmium & lead. Indian J. Med. Res. 2008;128(4):335–352. [PubMed] [Google Scholar]
- 155.Bhattacharya S. Protective role of the essential trace elements in the obviation of cadmium toxicity: glimpses of mechanisms. Biol. Trace Elem. Res. 2022;200(5):2239–2246. doi: 10.1007/s12011-021-02827-7. [DOI] [PubMed] [Google Scholar]
- 156.Bolkent S., Koyuturk M., Bulan O.K., Tunali S., Yanardag R., Tabakoglu A.O. The effects of combined α-tocopherol, ascorbic acid, and selenium against cadmium toxicity in rat intestine. J. Environ. Pathol. Toxicol. Oncol. 2007;26(1) doi: 10.1615/jenvironpatholtoxicoloncol.v26.i1.30. [DOI] [PubMed] [Google Scholar]
- 157.Flora S., Mittal M., Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008;128(4):501. [PubMed] [Google Scholar]
- 158.Sandbichler A.M., Höckner M. Cadmium protection strategies—a hidden trade-off? Int. J. Mol. Sci. 2016;17(1):139. doi: 10.3390/ijms17010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Unsal V., Dalkıran T., Çiçek M., Kölükçü E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv. Pharmaceut. Bull. 2020;10(2):184. doi: 10.34172/apb.2020.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Funakoshi T., Ueda K.-i., Shimada H., Kojima S. Effects of dithiocarbamates on toxicity of cadmium in rat primary hepatocyte cultures. Toxicology. 1997;116(1–3):99–107. doi: 10.1016/s0300-483x(96)03533-0. [DOI] [PubMed] [Google Scholar]
- 161.Haendel M.A., Tilton F., Bailey G.S., Tanguay R.L. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish. Toxicol. Sci. 2004;81(2):390–400. doi: 10.1093/toxsci/kfh202. [DOI] [PubMed] [Google Scholar]
- 162.Fatemi S.N., Tubafard S., Fatemi S.J., Nadi B. Evaluation of ATC as an orally administered drug in treatment of cadmium toxicity of rat organs. E-Journal of Chemistry. 2009;6(2):504–510. [Google Scholar]
- 163.Almeer R.S., AlBasher G.I., Alarifi S., Alkahtani S., Ali D., Abdel Moneim A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-42368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bjørklund G., Crisponi G., Nurchi V.M., Cappai R., Buha Djordjevic A., Aaseth J. A review on coordination properties of thiol-containing chelating agents towards mercury, cadmium, and lead. Molecules. 2019;24(18):3247. doi: 10.3390/molecules24183247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhattacharya S. The role of probiotics in the amelioration of cadmium toxicity. Biol. Trace Elem. Res. 2020;197(2):440–444. doi: 10.1007/s12011-020-02025-x. [DOI] [PubMed] [Google Scholar]
- 166.Mudhoo A., Sharma S.K., Garg V.K., Tseng C.-H. Arsenic: an overview of applications, health, and environmental concerns and removal processes. Crit. Rev. Environ. Sci. Technol. 2011;41(5):435–519. [Google Scholar]
- 167.Fatoki J.O., Badmus J.A. Arsenic as an environmental and human health antagonist: a review of its toxicity and disease initiation. Journal of Hazardous Materials Advances. 2022 [Google Scholar]
- 168.Raju N.J. Arsenic in the geo-environment: a review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111782. [DOI] [PubMed] [Google Scholar]
- 169.Wong C., Roberts S.M., Saab I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022;130 doi: 10.1016/j.yrtph.2022.105122. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y., Han Y.-H., Cao Y., Zhu Y.-G., Rathinasabapathi B., Ma L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Frontiers in plant science. 2017;8:268. doi: 10.3389/fpls.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Balali-Mood M., Riahi-Zanjani B., Mahdizadeh A., Moradi V., Fazeli-Bakhtiyari R. Arsenic and lead contaminations in commercial fruit juices of markets in Mashhad, Iran. Iranian Journal of Toxicology. 2018;12(3):15–20. [Google Scholar]
- 172.Mridha D., Gorain P.C., Joardar M., Das A., Majumder S., De A., Chowdhury N.R., Lama U., Pal R., Roychowdhury T. Rice grain arsenic and nutritional content during post harvesting to cooking: a review on arsenic bioavailability and bioaccessibility in humans. Food Res. Int. 2022 doi: 10.1016/j.foodres.2022.111042. [DOI] [PubMed] [Google Scholar]
- 173.Singh S., Rana S. Amelioration of arsenic toxicity by L-Ascorbic acid in laboratory rat. Journal of environmental biology. 2007;28(2):377. [PubMed] [Google Scholar]
- 174.Bhattacharya S. Medicinal plants and natural products in amelioration of arsenic toxicity: a short review. Pharm. Biol. 2017;55(1):349–354. doi: 10.1080/13880209.2016.1235207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ling S., Shan Q., Liu P., Feng T., Zhang X., Xiang P., Chen K., Xie H., Song P., Zhou L. Metformin ameliorates arsenic trioxide hepatotoxicity via inhibiting mitochondrial complex I. Cell Death Dis. 2017;8(11) doi: 10.1038/cddis.2017.482. e3159-e3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mandal D., Biswas S., Seal S., Mandal R., Das S., Basu A. Microbes and Microbial Biotechnology for Green Remediation. Elsevier; 2022. Arsenic toxicity and its clinical manifestations in Murshidabad district with some potential remedial measures; pp. 701–715. [Google Scholar]
- 177.Nasrin S., Kawser M., Ahmed S., Saha A.K., Haque A., Rahman R., Akter A., Islam S.N. Protective roles of some leafy and non-leafy vegetables against the severity of arsenic-induced skin lesions among women living in rural Bangladesh: a case control study. J. Bot. Res. 2022;4(1) [Google Scholar]
- 178.Chanda S., Roy J., Guha Mazumder D., Chakraborty T. Role of glutathione-S-transferase polymorphism on arsenic-induced protein carbonylation and urinary deoxy guanosine status. Exposure and Health. 2020;12:539–546. [Google Scholar]
- 179.Wang D., Liang Q., Tai D., Wang Y., Hao H., Liu Z., Huang L. Association of urinary arsenic with the oxidative DNA damage marker 8-hydroxy-2 deoxyguanosine: a meta-analysis. Sci. Total Environ. 2023;904 doi: 10.1016/j.scitotenv.2023.166600. [DOI] [PubMed] [Google Scholar]
- 180.Genchi G., Lauria G., Catalano A., Carocci A., Sinicropi M.S. Arsenic: a review on a great health issue worldwide. Appl. Sci. 2022;12(12):6184. [Google Scholar]
- 181.Hamza M., Alam S., Rizwan M., Naz A. "Health risks associated with arsenic contamination and its biotransformation mechanisms in environment: a review." hazardous environmental micro-pollutants. Health Impacts and Allied Treatment Technologies. 2022:241–288. [Google Scholar]
- 182.Chen Y., Wu F., Liu M., Parvez F., Slavkovich V., Eunus M., Ahmed A., Argos M., Islam T., Rakibuz-Zaman M. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environmental health perspectives. 2013;121(7):832–838. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lugo G., Cassady G., Palmisano P. Acute maternal arsenic intoxication with neonatal death. American journal of diseases of children. 1969;117(3):328–330. doi: 10.1001/archpedi.1969.02100030330015. [DOI] [PubMed] [Google Scholar]
- 184.Young E.G., Smith R.P. The arsenic content of hair and bone in acute and chronic arsenical poisoning. two cases examined posthumously from a medico-legal aspect. Br. Med. J. 1942;1(4233):251. [Google Scholar]
- 185.Gousios A.G., Adelson L. Electrocardiographic and radiographic findings in acute arsenic poisoning. Am. J. Med. 1959;27(4):659–663. doi: 10.1016/0002-9343(59)90049-x. [DOI] [PubMed] [Google Scholar]
- 186.Mealey J., Brownell G.L., Sweet W.H. Radioarsenic in plasma, urine, normal tissues, and intracranial neoplasms: distribution and turnover after intravenous injection in man. AMA Archives of Neurology & Psychiatry. 1959;81(3):310–320. doi: 10.1001/archneurpsyc.1959.02340150042005. [DOI] [PubMed] [Google Scholar]
- 187.Srivastava S., Flora S.J. Handbook of Toxicology of Chemical Warfare Agents. Elsevier; 2020. Arsenicals: toxicity, their use as chemical warfare agents, and possible remedial measures; pp. 303–319. [Google Scholar]
- 188.Soni R.K., Singh O. Hazardous Gases. Elsevier; 2021. Arsine: risk assessment, environmental, and health hazard; pp. 11–21. [Google Scholar]
- 189.Khanjani N., Jafarnejad A.-B., Tavakkoli L. Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev. Environ. Health. 2017;32(3):267–277. doi: 10.1515/reveh-2016-0068. [DOI] [PubMed] [Google Scholar]
- 190.Siddique A.E., Rahman M., Hossain M.I., Karim Y., Hasibuzzaman M., Biswas S., Islam M.S., Rahman A., Hossen F., Mondal V. Association between chronic arsenic exposure and the characteristic features of asthma. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125790. [DOI] [PubMed] [Google Scholar]
- 191.Zhao J., Li A., Mei Y., Zhou Q., Li Y., Li K., Xu Q. The association of arsenic exposure with hypertension and blood pressure: a systematic review and dose–response meta-analysis. Environmental Pollution. 2021;289 doi: 10.1016/j.envpol.2021.117914. [DOI] [PubMed] [Google Scholar]
- 192.Alshana U., Altun B., Ertaş N., Çakmak G., Kadioglu E., Hisarlı D., Aşık E., Atabey E., Çelebi C.R., Bilir N. Evaluation of low to moderate arsenic exposure, metabolism and skin lesions in a Turkish rural population exposed through drinking water. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.135277. [DOI] [PubMed] [Google Scholar]
- 193.Madrid E., Gonzalez-Miranda I., Muñoz S., Rejas C., Cardemil F., Martinez F., Cortes J.P., Berasaluce M., Párraga M. Arsenic concentration in topsoil of central Chile is associated with aberrant methylation of P53 gene in human blood cells: a cross-sectional study. Environ. Sci. Pollut. Control Ser. 2022:1–10. doi: 10.1007/s11356-022-19085-2. [DOI] [PubMed] [Google Scholar]
- 194.Xenakis J.G., Douillet C., Bell T.A., Hock P., Farrington J., Liu T., Murphy C.E., Saraswatula A., Shaw G.D., Nativio G. An interaction of inorganic arsenic exposure with body weight and composition on type 2 diabetes indicators in Diversity Outbred mice. Mamm. Genome. 2022;33(4):575–589. doi: 10.1007/s00335-022-09957-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang X., Karvonen-Gutierrez C.A., Herman W.H., Mukherjee B., Park S.K. Metals and risk of incident metabolic syndrome in a prospective cohort of midlife women in the United States. Environ. Res. 2022;210 doi: 10.1016/j.envres.2022.112976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kunwittaya S., Ruksee N., Khamnong T., Jiawiwatkul A., Kleebpung N., Chumchua V., Plitponkarnpim A., Nopparat C., Permpoonputtana K. Inorganic arsenic contamination and the health of children living near an inactive mining site: northern Thailand. EXCLI journal. 2022;21:1007–1014. doi: 10.17179/excli2022-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saxena R., Gamble M., Wasserman G.A., Liu X., Parvez F., Navas-Acien A., Islam T., Factor-Litvak P., Uddin M.N., Kioumourtzoglou M.-A. Mixed metals exposure and cognitive function in Bangladeshi adolescents. Ecotoxicol. Environ. Saf. 2022;232 doi: 10.1016/j.ecoenv.2022.113229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ratnaike R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003;79(933):391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Greenberg M.I. Diagnosis and Management of the Critically Poisoned Patient. Taylor & Francis; 2018. Critical care toxicology. [Google Scholar]
- 200.Agarwal A., Kn S.K., Jorwal P., Quadri J.A., Gupta G., Biswas A. Arsenic intoxication with renal failure managed with hemodialysis alone: a case report. Drug Discoveries & Therapeutics. 2022;16(1):49–51. doi: 10.5582/ddt.2021.01110. [DOI] [PubMed] [Google Scholar]
- 201.Mahieu P., Buchet J.-P., Roels H., Lauwerys R. The metabolism of arsenic in humans acutely intoxicated by As2O3. Its significance for the duration of BAL therapy. Clin. Toxicol. 1981;18(9):1067–1075. doi: 10.3109/15563658108990336. [DOI] [PubMed] [Google Scholar]
- 202.Fournier L., Thomas G., Garnier R., Buisine A., Houze P., Pradier F., Dally S. 2, 3-Dimercaptosuccinic acid treatment of heavy metal poisoning in humans. Medical Toxicology and Adverse Drug Experience. 1988;3(6):499–504. doi: 10.1007/BF03259898. [DOI] [PubMed] [Google Scholar]
- 203.Mückter H., Lieb B., Reich F.-X., Hunder G., Walther U., Fichtl B. Are we ready to replace dimercaprol (BAL) as an arsenic antidote? Hum. Exp. Toxicol. 1997;16(8):460–465. doi: 10.1177/096032719701600807. [DOI] [PubMed] [Google Scholar]
- 204.Kalia K., Flora S.J. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. Journal of occupational health. 2005;47(1):1–21. doi: 10.1539/joh.47.1. [DOI] [PubMed] [Google Scholar]
- 205.Flora S., Flora G., Saxena G., Mishra M. Arsenic and lead induced free radical generation and their reversibility following chelation. Cellular and molecular biology. 2007;53(1):26–47. [PubMed] [Google Scholar]
- 206.Flora S.J., Bhadauria S., Pachauri V., Yadav A. Monoisoamyl 2, 3‐dimercaptosuccinic acid (MiADMSA) demonstrates higher efficacy by oral route in reversing arsenic toxicity: a pharmacokinetic approach. Basic Clin. Pharmacol. Toxicol. 2012;110(5):449–459. doi: 10.1111/j.1742-7843.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 207.Chen H.-C., Chiou H.-Y.C., Tsai M.-L., Chen S.-C., Lin M.-H., Chuang T.-C., Hung C.-H., Kuo C.-H. Effects of montelukast on arsenic-induced epithelial-mesenchymal transition and the role of reactive oxygen species production in human bronchial epithelial cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.877125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abdollahzade N., Mihanfar A., Majidinia M. Molecular mechanisms underlying ameliorative impact of melatonin against age‐dependent chronic arsenic toxicity in rats’ brains. J. Exp. Zool. Part A: Ecological and Integrative Physiology. 2022;337(9–10):1010–1024. doi: 10.1002/jez.2603. [DOI] [PubMed] [Google Scholar]
- 209.Acharyya N., Chattopadhyay S., Maiti S. Chemoprevention against arsenic-induced mutagenic DNA breakage and apoptotic liver damage in rat via antioxidant and SOD1 upregulation by green tea (Camellia sinensis) which recovers broken DNA resulted from arsenic-H2O2 related in vitro oxidant stress. Journal of Environmental Science and Health, Part C. 2014;32(4):338–361. doi: 10.1080/10590501.2014.967061. [DOI] [PubMed] [Google Scholar]
- 210.Ye T., Zhen J., Du Y., Zhou J.K., Peng A., Vaziri N.D., Mohan C., Xu Y., Zhou X.J. Green tea polyphenol (−)-epigallocatechin-3-gallate restores Nrf2 activity and ameliorates crescentic glomerulonephritis. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vineetha V.P., Girija S., Soumya R.S., Raghu K.G. Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Funct. 2014;5(3):502–511. doi: 10.1039/c3fo60470e. [DOI] [PubMed] [Google Scholar]
- 212.Khatun S., Maity M., Perveen H., Dash M., Chattopadhyay S. Spirulina platensis ameliorates arsenic-mediated uterine damage and ovarian steroidogenic disorder. Facets. 2018;3(1):736–753. [Google Scholar]
- 213.Famurewa A.C., Maduagwuna E., Aloke C., Azubuike-Osu S.O., Narayanankutty A. Virgin coconut oil ameliorates arsenic hepatorenal toxicity and NO-mediated inflammation via suppression of oxidative stress in rats (TJPS-2021-0292. R1) Thai Journal of Pharmaceutical Sciences (TJPS) 2022;46(2) [Google Scholar]
- 214.Panda S.K., Kumar D., Jena G.R., Patra R.C., Panda S.K., Sethy K., Mishra S.K., Swain B.K., Naik P.K., Beura C.K. Hepatorenal toxicity of inorganic arsenic in white pekin ducks and its amelioration by using ginger. Biol. Trace Elem. Res. 2022:1–20. doi: 10.1007/s12011-022-03317-0. [DOI] [PubMed] [Google Scholar]
- 215.Kumar A., Kumar V., Akhouri V., Kumar R., Ali M., Rashmi T., Chand G.B., Singh S.K., Ghosh A.K. Protective efficacy of Coriandrum sativum seeds against arsenic induced toxicity in Swiss albino mice. Toxicol. Res. 2022:1–11. doi: 10.1007/s43188-022-00123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Akinboro A., Adedosu O.T., Badmus J.A., Adegbola P.I., Abubakar M.Y., James R.O., Aborisade A.B. Aspalathus linearis extract ameliorate haematological disorder, dyslipidaemia and tissue toxicity associated with arsenic exposure in rats. Phytomedicine. 2022;2(1) [Google Scholar]
- 217.Bjørklund G., Rahaman M.S., Shanaida M., Lysiuk R., Oliynyk P., Lenchyk L., Chirumbolo S., Chasapis C.T., Peana M. Natural dietary compounds in the treatment of arsenic toxicity. Molecules. 2022;27(15):4871. doi: 10.3390/molecules27154871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ghabaee D.N.Z., Amiri F.T., Moghaddam A.E., Khalatbary A.R., Zargari M. Administration of zinc against arsenic-induced nephrotoxicity during gestation and lactation in rat model. Journal of Nephropathology. 2017;6(2):74. doi: 10.15171/jnp.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xing P., Zhang Y., Chi Q., Li S. Zinc alleviates arsenic-induced inflammation and apoptosis in the head kidney of common carp by inhibiting oxidative stress and endoplasmic reticulum stress. Biol. Trace Elem. Res. 2022;200(5):2380–2390. doi: 10.1007/s12011-021-02837-5. [DOI] [PubMed] [Google Scholar]
- 220.Bhardwaj H., Singh C., Nayyar S., Sodhi S., Jindal R. Effect of vitamin E and selenium (SE) supplementation on biochemical parameters and expression of metallothionein (MT-2) in heavy metals exposed buffaloes. Indian J. Anim. Res. 2022;1:9. [Google Scholar]
- 221.Das S., Majumder B., Biswas A.K. Selenium alleviates arsenic induced stress by modulating growth, oxidative stress, antioxidant defense and thiol metabolism in rice seedlings. Int. J. Phytoremediation. 2022;24(7):763–777. doi: 10.1080/15226514.2021.1975639. [DOI] [PubMed] [Google Scholar]
- 222.Samad N., Rao T., Bhatti S.A., Imran I. Inhibitory effects of selenium on arsenic-induced anxiety-/depression-like behavior and memory impairment. Biol. Trace Elem. Res. 2022;200(2):689–698. doi: 10.1007/s12011-021-02679-1. [DOI] [PubMed] [Google Scholar]
- 223.Gamble M.V., Liu X., Ahsan H., Pilsner J.R., Ilievski V., Slavkovich V., Parvez F., Levy D., Factor-Litvak P., Graziano J.H. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environmental health perspectives. 2005;113(12):1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gamble M.V., Liu X., Ahsan H., Pilsner J.R., Ilievski V., Slavkovich V., Parvez F., Chen Y., Levy D., Factor-Litvak P. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. The American journal of clinical nutrition. 2006;84(5):1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pilsner J.R., Liu X., Ahsan H., Ilievski V., Slavkovich V., Levy D., Factor-Litvak P., Graziano J.H., Gamble M.V. Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. The American journal of clinical nutrition. 2007;86(4):1179–1186. doi: 10.1093/ajcn/86.4.1179. [DOI] [PubMed] [Google Scholar]
- 226.Pilsner J.R., Liu X., Ahsan H., Ilievski V., Slavkovich V., Levy D., Factor-Litvak P., Graziano J.H., Gamble M.V. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environmental health perspectives. 2009;117(2):254–260. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zablotska L.B., Chen Y., Graziano J.H., Parvez F., van Geen A., Howe G.R., Ahsan H. Protective effects of B vitamins and antioxidants on the risk of arsenic-related skin lesions in Bangladesh. Environmental health perspectives. 2008;116(8):1056–1062. doi: 10.1289/ehp.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gopalkrishnan A., Rao M.V. Amelioration by vitamin A upon arsenic induced metabolic and neurotoxic effects. Journal of health science. 2006;52(5):568–577. [Google Scholar]
- 229.Cheraghi E., Piroozmanesh H., Soleimani Mehranjani M. The effects of vitamin C on the quality of semen, testicular tissue, and apoptosis in mice exposed to sodium arsenite. Pharmaceutical and Biomedical Research. 2022;8(1):13–22. [Google Scholar]
- 230.Harsin A.O., Firozian F., Fereshteh M., Ranjbar A. Evaluation of ascorbic acid niosomes as potential detoxifiers in oxidative stress-induced HEK-293 cells by arsenic trioxide. Iran. J. Pharm. Res. (IJPR) 2022 doi: 10.5812/ijpr-127038. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mittal M., Flora S. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug and chemical toxicology. 2007;30(3):263–281. doi: 10.1080/01480540701380075. [DOI] [PubMed] [Google Scholar]
- 232.Mondal R., Biswas S., Chatterjee A., Mishra R., Mukhopadhyay A., Bhadra R.K., Mukhopadhyay P.K. Protection against arsenic-induced hematological and hepatic anomalies by supplementation of vitamin C and vitamin E in adult male rats. J. Basic Clin. Physiol. Pharmacol. 2016;27(6):643–652. doi: 10.1515/jbcpp-2016-0020. [DOI] [PubMed] [Google Scholar]
- 233.Mir U.Z., Rashid A., Yousaf M.J., Khan H.G., Mir A., Laique T. Synergistic efficacy of the combination of Co Q10 and α-tocopherol against arsenic induced hepatotoxicity in sprague dawley rats. Pakistan Journal of Medical & Health Sciences. 2022;16(2) 77-77. [Google Scholar]
- 234.Verret W.J., Chen Y., Ahmed A., Islam T., Parvez F., Kibriya M.G., Graziano J.H., Ahsan H. A randomized, double-blind placebo-controlled trial evaluating the effects of vitamin E and selenium on arsenic-induced skin lesions in Bangladesh. Journal of occupational and environmental medicine. 2005:1026–1035. doi: 10.1097/01.jom.0000183095.45050.97. [DOI] [PubMed] [Google Scholar]
- 235.Sanyal T., Bhattacharjee P., Paul S., Bhattacharjee P. Recent advances in arsenic research: significance of differential susceptibility and sustainable strategies for mitigation. Front. Public Health. 2020;8:464. doi: 10.3389/fpubh.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liao K.-W., Chien L.-C., Chen Y.-C., Kao H.-C. Sex-specific differences in early renal impairment associated with arsenic, lead, and cadmium exposure among young adults in Taiwan. Environ. Sci. Pollut. Control Ser. 2022:1–10. doi: 10.1007/s11356-022-19521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Khandker S., Dey R.K., Islam A.M., Ahmad S.A., Al Mahmud I. Arsenic-safe drinking water and antioxidants for the management of arsenicosis patients. ||| Bangladesh Journal of Pharmacology. 2006;1(2):42–50. [Google Scholar]
- 238.Misbahuddin M., Bashar T., Hossain M.A. Effectiveness of garlic oil in the treatment of arsenical palmar keratosis. ||| Bangladesh Journal of Pharmacology. 2013;8(1):22–27. [Google Scholar]
- 239.Khan S.S., Sharma A., Flora S.J., Phytochemicals in the management of arsenic toxicity, Chem. Res. Toxicol. (2022) 16;35(6):916-34. [DOI] [PubMed]
- 240.Kushwaha P., Yadav A., Samim M., Flora S. Combinatorial drug delivery strategy employing nano-curcumin and nano-MiADMSA for the treatment of arsenic intoxication in mouse. Chem. Biol. Interact. 2018;286:78–87. doi: 10.1016/j.cbi.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 241.Bhalla A., Pannu A. Are Ayurvedic medications store house of heavy metals? Toxicology Research. 2022;11(1):179–183. doi: 10.1093/toxres/tfab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Paul N.P., Galván A.E., Yoshinaga-Sakurai K., Rosen B.P., Yoshinaga M. Arsenic in medicine: past, present and future. Biometals. 2022:1–19. doi: 10.1007/s10534-022-00371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Flora S. Challenges in the field of toxicity of metals in India including the development of new treatment. Journal of Environmental Biology. 2022;43(4):III–V. [Google Scholar]
- 244.Taher A.T., Saliba A.N., Kuo K.H., Giardina P.J., Cohen A.R., Neufeld E.J., Aydinok Y., Kwiatkowski J.L., Jeglinski B.I., Pietropaolo K. Safety and pharmacokinetics of the oral iron chelator SP‐420 in β‐thalassemia. Am. J. Hematol. 2017;92(12):1356–1361. doi: 10.1002/ajh.24914. [DOI] [PubMed] [Google Scholar]
- 245.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 246.Flora S.J., Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7(7):2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yao M., Guo X., Shao X., Wei Y., Zhang X., Wang H., Xu F. Soluble dietary fiber from Prunus persica dregs alleviates gut microbiota dysfunction through lead excretion. Food Chem. Toxicol. 2023;175 doi: 10.1016/j.fct.2023.113725. [DOI] [PubMed] [Google Scholar]
- 248.Yadav A., Mathur R., Samim M., Lomash V., Kushwaha P., Pathak U., Babbar A.K., Singh Flora S.J., Mishra A.K., Kaushik M.P. Nanoencapsulation of DMSA monoester for better therapeutic efficacy of the chelating agent against arsenic toxicity. Nanomedicine. 2014;9(4):465–481. doi: 10.2217/NNM.13.17. [DOI] [PubMed] [Google Scholar]
- 249.Jurasekova Z., Torreggiani A., Tamba M., Sanchez-Cortes S., Garcia-Ramos J. Raman and surface-enhanced Raman scattering (SERS) investigation of the quercetin interaction with metals: evidence of structural changing processes in aqueous solution and on metal nanoparticles. J. Mol. Struct. 2009;918(1–3):129–137. [Google Scholar]
- 250.Yaqoob A., Rehman D., Akash M.S.H. Biochemical profiling of metabolomics in heavy metal-intoxicated impaired metabolism and its amelioration using plant-based bioactive compound. Front. Mol. Biosci. 2022;1149 doi: 10.3389/fmolb.2022.1029729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mishra D., Flora S. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol. Trace Elem. Res. 2008;122(2):137–147. doi: 10.1007/s12011-007-8064-9. [DOI] [PubMed] [Google Scholar]
- 252.Cai P., Zhu Q., Cao Q., Bai Y., Zou H., Gu J., Yuan Y., Liu X., Liu Z., Bian J. Quercetin and allicin can alleviate the hepatotoxicity of lead (Pb) through the PI3K signaling pathway. J. Agric. Food Chem. 2021;69(32):9451–9460. doi: 10.1021/acs.jafc.1c03794. [DOI] [PubMed] [Google Scholar]
- 253.Bu T., Mi Y., Zeng W., Zhang C. Protective effect of quercetin on cadmium‐induced oxidative toxicity on germ cells in male mice. Anat. Rec.: Advances in Integrative Anatomy and Evolutionary Biology. 2011;294(3):520–526. doi: 10.1002/ar.21317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are outlined in the current review either as tables, figures, and context references.