Abstract
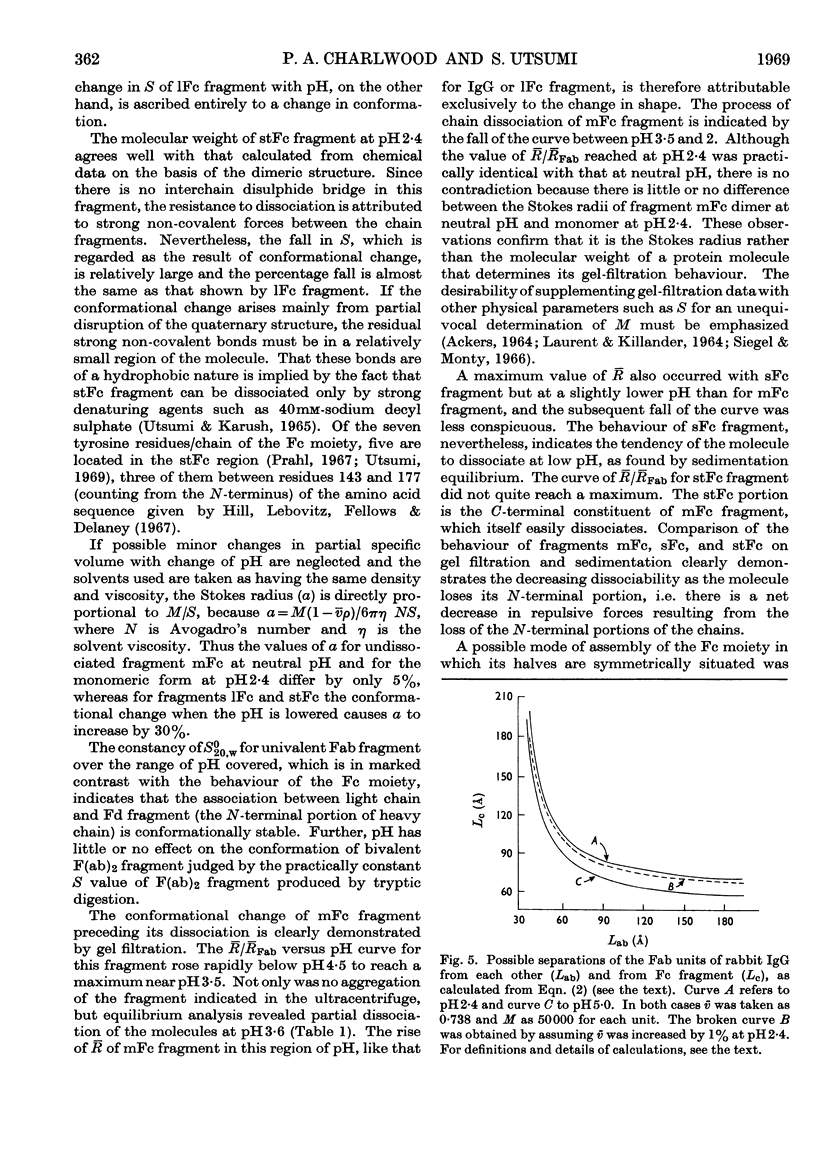
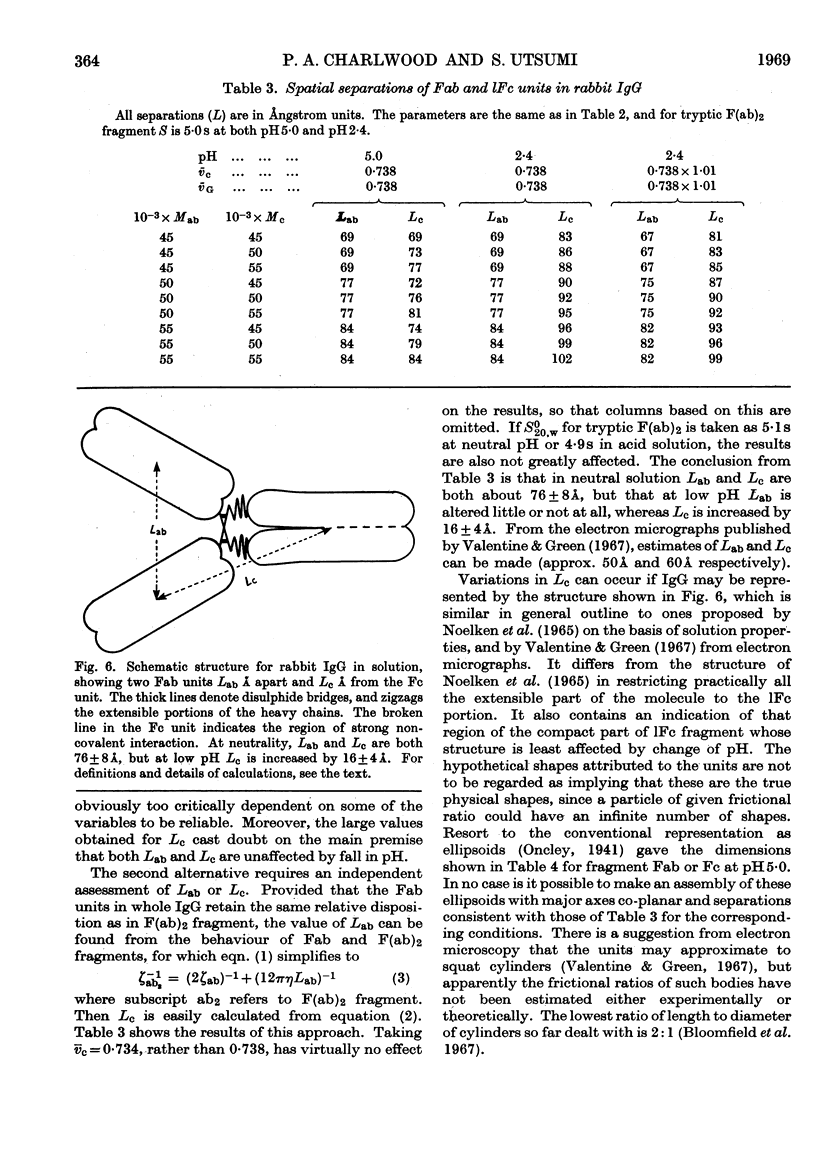
1. The sedimentation coefficients of rabbit immunoglobulin G, four types of Fc fragments, univalent Fab and bivalent F(ab)2 fragments were measured as a function of pH. 2. In conjunction with molecular-weight determinations by sedimentation equilibrium, and with the behaviour on gel filtration, this enabled the state of association of the Fc fragments to be followed. 3. The type possessing an interchain disulphide bond, 1Fc fragment, changed extensively in structure, but not in molecular weight. 4. There was good correlation between the readiness to dissociate and the chain length of the shorter Fc fragments that do not contain the interchain covalent bond. 5. The increasing resistance to dissociation as the fragments became shorter ran parallel with the ability to resist enzymic attack. 6. The site of the strong association between component chains of Fc fragment is located in the C-terminal half. 7. The gel-filtration behaviour of the Fc fragments clearly confirms that the process is governed by the Stokes radius rather than molecular weight. 8. The ultracentrifugal results were used to estimate the separations of the hydrodynamic subunits in intact immunoglobulin G, and as a basis for a schematic structure.
Full text
PDF

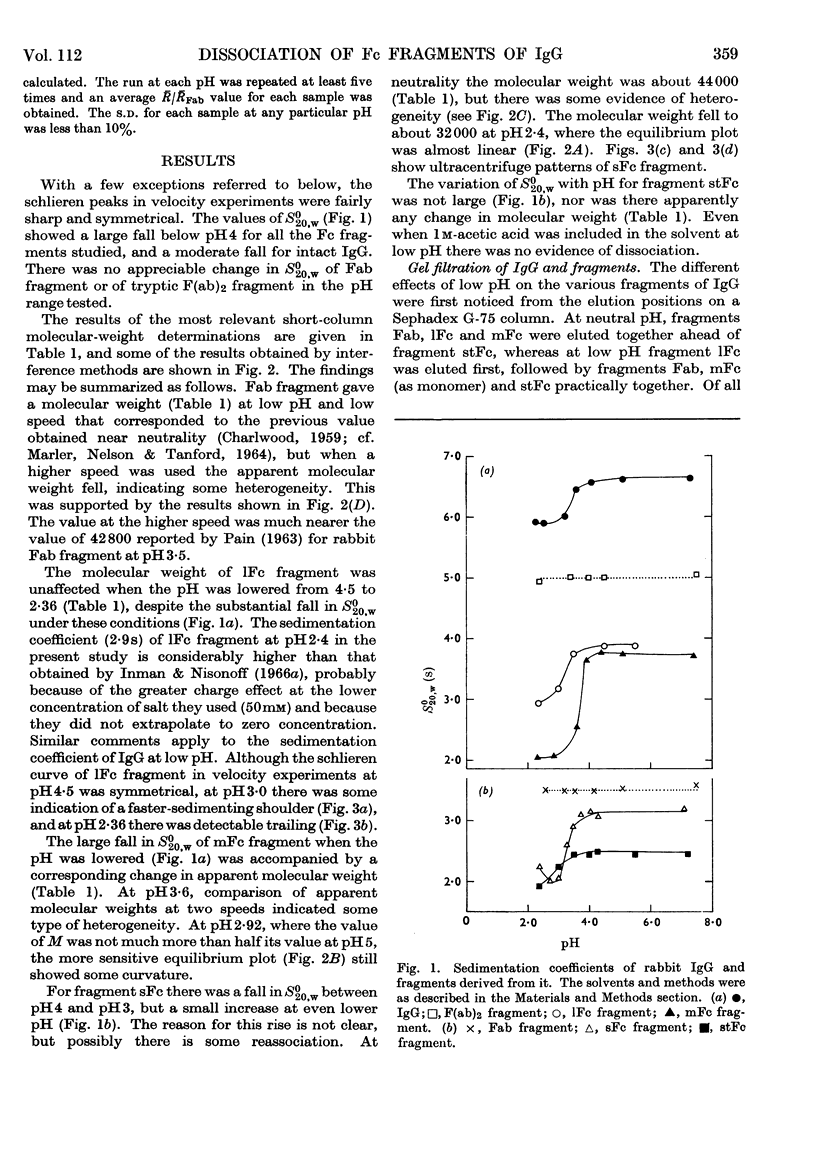

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Adams E. T., Jr Sedimentation equilibrium in reacting systems. 3. Evaluation of the number average (Mn(c)) molecular weight, equilibrium constants, and nonideal effects. Biochemistry. 1965 Aug;4(8):1646–1654. doi: 10.1021/bi00884a029. [DOI] [PubMed] [Google Scholar]
- Bloomfield V., Dalton W. O., Van Holde K. E. Frictional coefficients of multisubunit structures. I. Theory. Biopolymers. 1967 Feb;5(2):135–148. doi: 10.1002/bip.1967.360050202. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. The structure of bovine serum albumin at low pH. Biochemistry. 1966 Feb;5(2):684–689. doi: 10.1021/bi00866a039. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. NEW POSSIBILITIES IN THE DESIGN OF ULTRACENTRIFUGAL EQUILIBRIUM EXPERIMENTS. Biochem Biophys Res Commun. 1965 Apr 9;19:243–248. doi: 10.1016/0006-291x(65)90512-7. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Ultracentrifugal examination of digestion products from rabbit gamma-globulin. Biochem J. 1959 Sep;73:126–127. [PubMed] [Google Scholar]
- Charlwood P. A. Transient distributions ensuing when ultracentrifugal equilibrium is disturbed by a substantial increase in speed. Can J Biochem. 1968 Aug;46(8):845–850. doi: 10.1139/o68-126. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Inman F. P., Nisonoff A. Localization of noncovalent interactions between the heavy chains of rabbit gamma-G-globulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):542–549. doi: 10.1073/pnas.56.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman F. P., Nisonoff A. Reversible dissociation of fragment Fc of rabbit gamma-G-immunoglobulin. J Biol Chem. 1966 Jan 25;241(2):322–329. [PubMed] [Google Scholar]
- MARLER E., NELSON C. A., TANFORD C. THE POLYPEPTIDE CHAINS OF RABBIT GAMMA-GLOBULIN AND ITS PAPAIN-CLEAVED FRAGMENTS. Biochemistry. 1964 Feb;3:279–284. doi: 10.1021/bi00890a024. [DOI] [PubMed] [Google Scholar]
- NOELKEN M. E., NELSON C. A., BUCKLEY C. E., 3rd, TANFORD C. GROSS CONFORMATION OF RABBIT 7 S GAMMA-IMMUNOGLOBULIN AND ITS PAPAIN-CLEAVED FRAGMENTS. J Biol Chem. 1965 Jan;240:218–224. [PubMed] [Google Scholar]
- PAIN R. H. THE MOLECULAR WEIGHTS OF THE PEPTIDE CHAINS OF GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:234–239. doi: 10.1042/bj0880234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER J. L., NISONOFF A., VANHOLDE K. E. DISSOCIATION OF RABBIT GAMMA GLOBULIN INTO SUBUNITS BY REDUCTION AND ACIDIFICATION. Proc Natl Acad Sci U S A. 1963 Aug;50:314–321. doi: 10.1073/pnas.50.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHELPS R. A., CANN J. R. On the modification of gamma-globulin by acid. Biochim Biophys Acta. 1957 Jan;23(1):149–154. doi: 10.1016/0006-3002(57)90297-4. [DOI] [PubMed] [Google Scholar]
- Prahl J. W. Enzymic degradation of the Fc fragment of rabbit immunoglobulin IgG. Biochem J. 1967 Aug;104(2):647–655. doi: 10.1042/bj1040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Thorpe N. O., Deutsch H. F. Studies on papain produced subunits of human gamma-G-globulins. I. Physicochemical and immunochemical properties of gamma-G-globulin Fc-fragments. Immunochemistry. 1966 Jul;3(4):317–327. doi: 10.1016/0019-2791(66)90093-0. [DOI] [PubMed] [Google Scholar]
- Utsumi S. Stepwise cleavage of rabbit immunoglobulin G by papain and isolation of four types of biologically active Fc fragments. Biochem J. 1969 Apr;112(3):343–355. doi: 10.1042/bj1120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J., Allison A. C. Thin-layer Sephadex chromatography and immunodiffusion. Lancet. 1967 Jul 15;2(7507):123–126. doi: 10.1016/s0140-6736(67)92963-7. [DOI] [PubMed] [Google Scholar]