Abstract
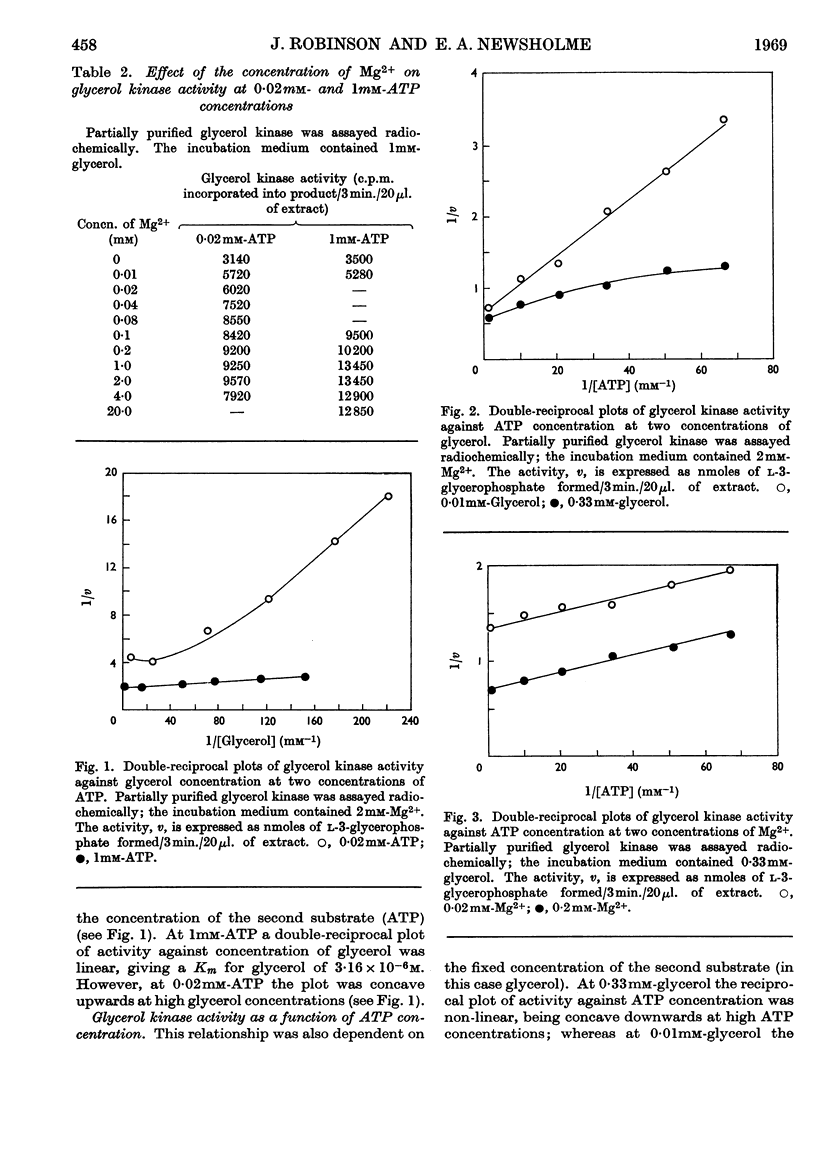
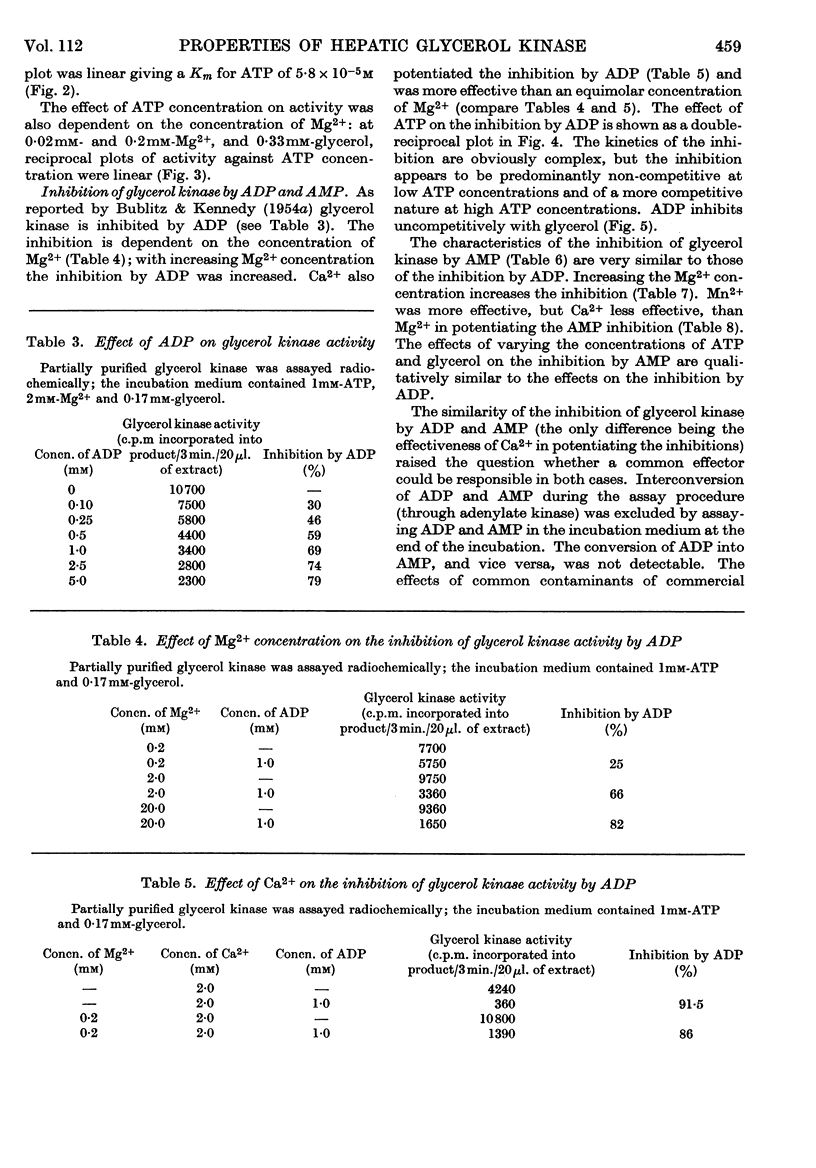
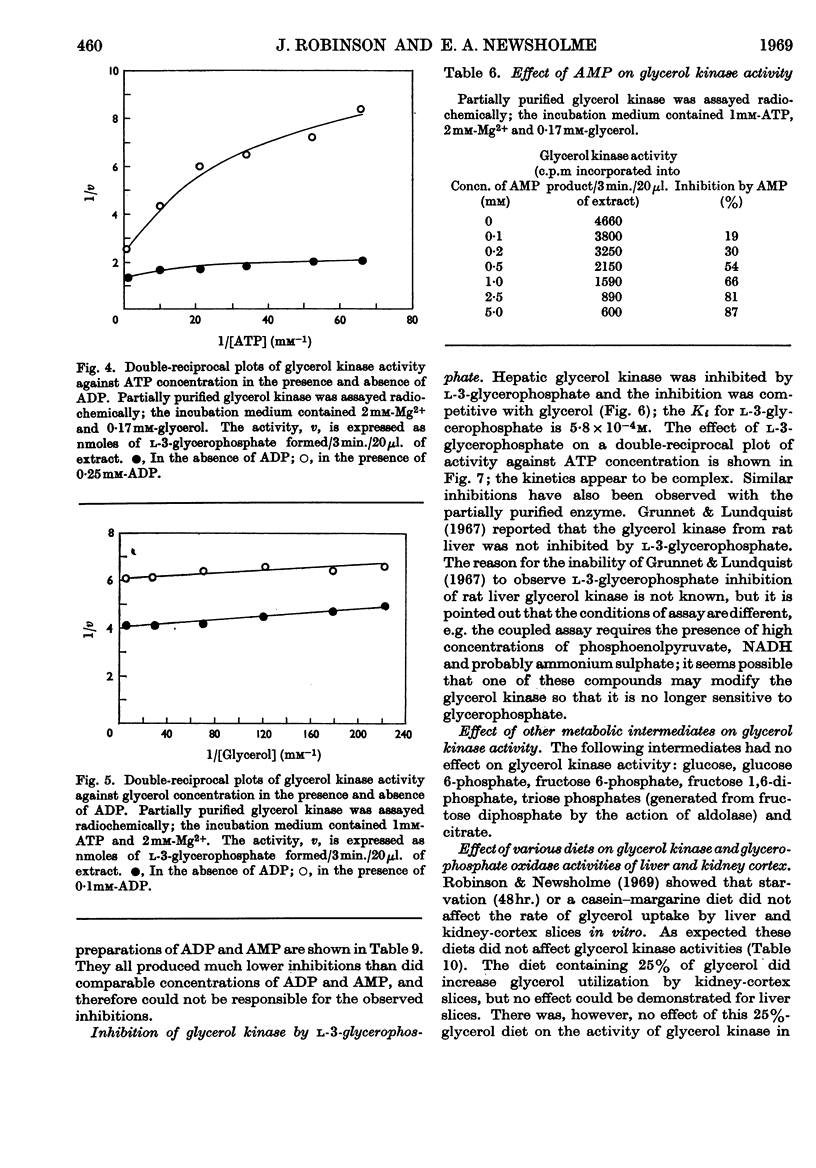
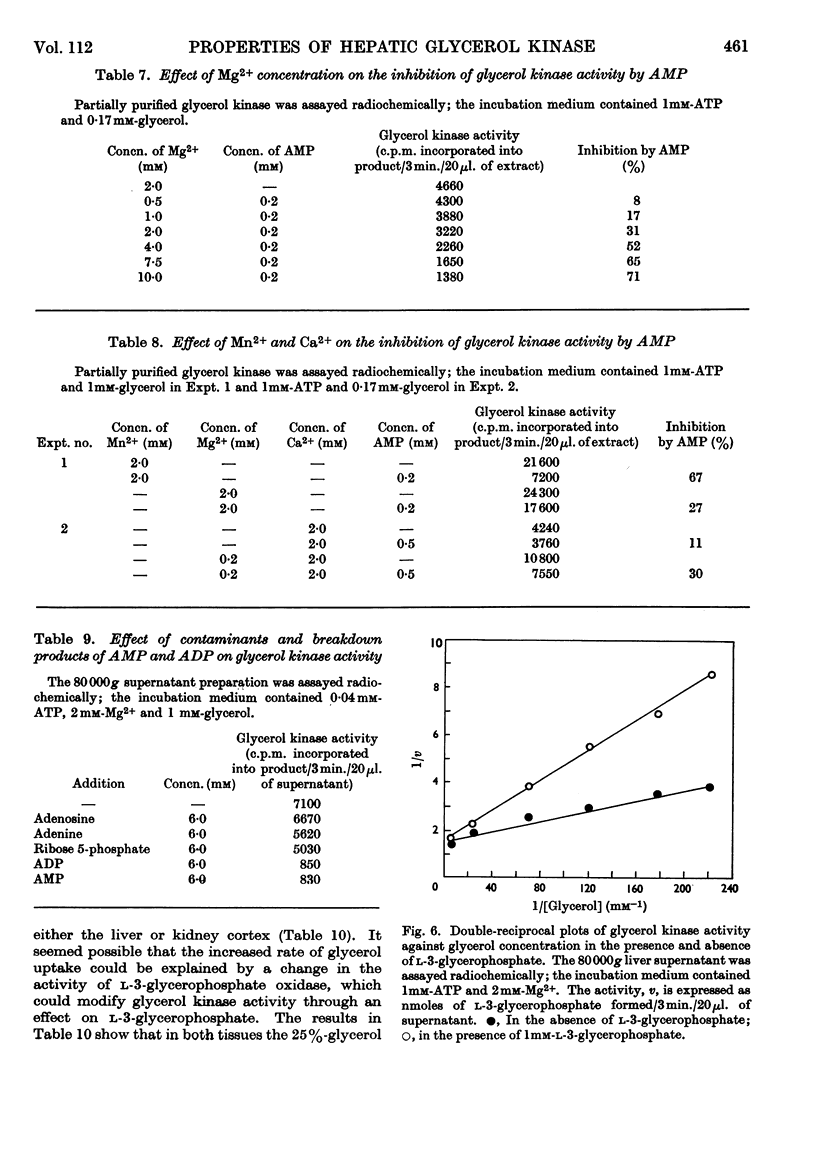
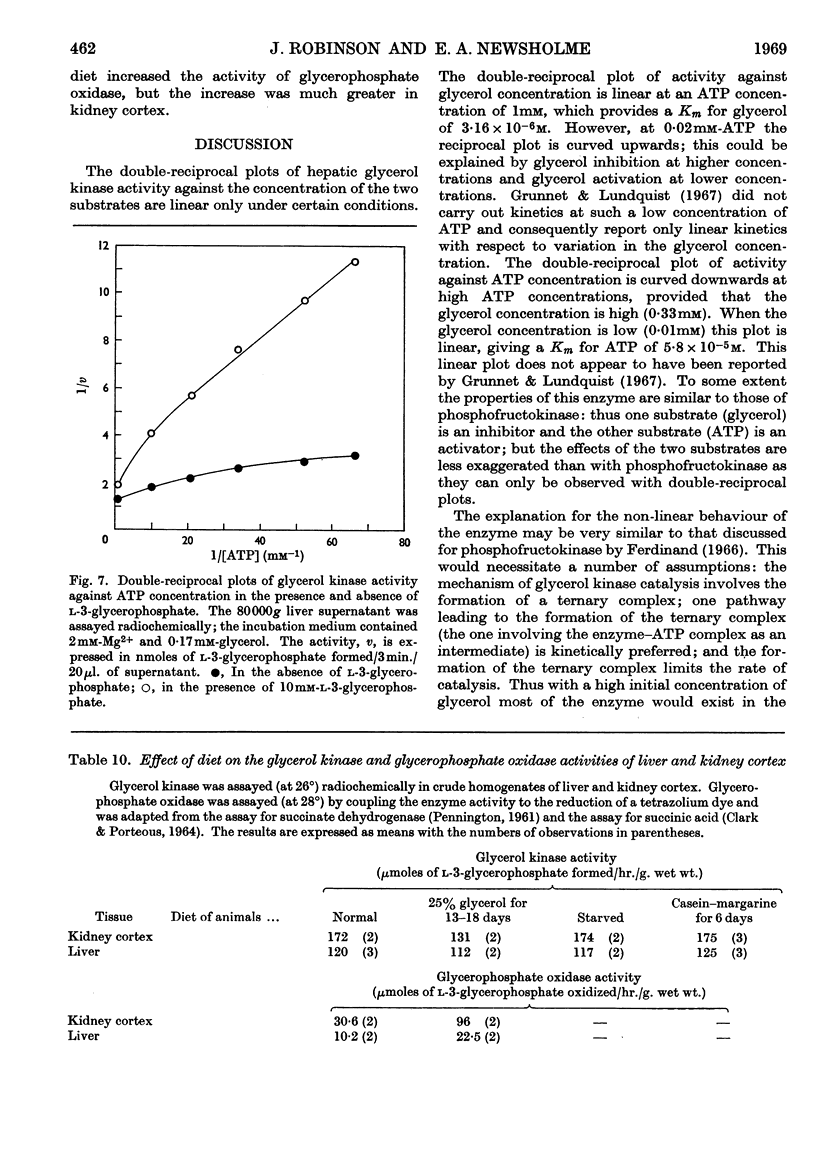
1. Glycerol kinase (EC 2.7.1.30) is shown to catalyse a non-equilibrium reaction in rat liver; and, as it is the first enzyme in the pathway metabolizing glycerol, its properties may be pertinent to the metabolic regulation of glycerol uptake and utilization by this tissue. 2. The properties of hepatic glycerol kinase were studied by using a radiochemical technique to measure the enzyme activity. When the concentration of ATP is low the activity of glycerol kinase is inhibited by high concentrations of glycerol; but when the concentration of ATP is high there is no inhibition and the double-reciprocal plot is linear, providing a Km for glycerol of 3·16×10−6m. Glycerol kinase is activated by high ATP concentrations provided that the concentration of the second substrate (glycerol) is high; at low concentrations of glycerol ATP does not activate the enzyme so that the double-reciprocal plot is linear, providing a Km for ATP of 5·8×10−5m. It is suggested that these kinetics may be explained by a model similar to that described by Ferdinand (1966) for phosphofructokinase. 3. Hepatic glycerol kinase is inhibited by ADP and AMP, and raising the Mg2+ concentration increases the inhibition by these two compounds; this suggests that ADP–Mg2+ and AMP–Mg2+ complexes are the inhibitory species. The physiological significance of these inhibitions may be to prevent phosphorylation of glycerol when the hepatic ATP concentration is low. It is suggested that this inhibition may provide an approach to the problem of measurement of rates of lipolysis by glycerol release in tissues that contain glycerol kinase (e.g. liver, kidney, muscle, adipose tissue). 4. Hepatic glycerol kinase is inhibited by l-3-glycerophosphate competitively with respect to glycerol. The physiological significance of this inhibition may be that factors that change the intracellular concentration of l-3-glycerophosphate could change glycerol uptake by the tissue. Thus it is suggested that thyroxine treatment or feeding rats on a diet high in glycerol, which increase the activity of glycerophosphate oxidase in liver and kidney cortex respectively, lead to an increased glycerol uptake through a decrease in the concentration of glycerophosphate in these tissues. It is known that ethanol administration decreases glycerol uptake by liver, and this can be explained by the increased concentration of l-3-glycerophosphate causing inhibition of glycerol kinase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORCHGREVINK C. F., HAVEL R. J. TRANSPORT OF GLYCEROL IN HUMAN BLOOD. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:946–949. doi: 10.3181/00379727-113-28539. [DOI] [PubMed] [Google Scholar]
- BUBLITZ C., KENNEDY E. P. A note of the asymmetrical metabolism of glycerol. J Biol Chem. 1954 Dec;211(2):963–967. [PubMed] [Google Scholar]
- BUBLITZ C., KENNEDY E. P. Synthesis of phosphatides in isolated mitochondria. III. The enzymatic phosphorylation of glycerol. J Biol Chem. 1954 Dec;211(2):951–961. [PubMed] [Google Scholar]
- CAHILL G. F., Jr, ASHMORE J., RENOLD A. E., HASTINGS A. B. Blood glucose and the liver. Am J Med. 1959 Feb;26(2):264–282. doi: 10.1016/0002-9343(59)90316-x. [DOI] [PubMed] [Google Scholar]
- Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. A possible mechanism for phosphofructokinase. Biochem J. 1966 Jan;98(1):278–283. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland R. A., Krebs H. A. The effect of thyroxine treatment on the rate of gluconeogenesis in the perfused rat liver. Biochem J. 1967 Sep;104(3):45P–45P. [PMC free article] [PubMed] [Google Scholar]
- Grunnet N., Lundquist F. Kinetics of glycerol kinases from mammalian liver and Candida mycoderma. Eur J Biochem. 1967 Dec;3(1):78–84. doi: 10.1111/j.1432-1033.1967.tb19500.x. [DOI] [PubMed] [Google Scholar]
- HOBERMAN H. D., D'ADAMO A., Jr Coupling of oxidation of substrates to reductive biosyntheses. VI. Studies with glycerol-2-D. J Biol Chem. 1960 Jun;235:1599–1602. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Krebs H. A. The effects of ethanol on the metabolic activities of the liver. Adv Enzyme Regul. 1968;6:467–480. doi: 10.1016/0065-2571(68)90029-0. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., TAKEMORI A. E., LARDY H. Enhanced oxidation of alpha-glycerophosphate by mitochondria of thyroid-fed rats. J Biol Chem. 1959 Nov;234:3051–3054. [PubMed] [Google Scholar]
- Lundquist F., Tygstrup N., Winkler K., Jensen K. B. Glycerol metabolism in the human liver: inhibition by ethanol. Science. 1965 Oct 29;150(3696):616–617. doi: 10.1126/science.150.3696.616. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Rolleston F. S., Taylor K. Factors affecting the glucose 6-phosphate inhibition of hexokinase from cerebral cortex tissue of the guinea pig. Biochem J. 1968 Jan;106(1):193–201. doi: 10.1042/bj1060193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Newsholme E. A. Inhibition of liver glycerol kinase by adenosine monophosphate and L-alpha-glycerophosphate. Biochem J. 1967 Sep;104(3):70P–70P. [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Newsholme E. A. The effects of dietary conditions and glycerol concentration on glycerol uptake by rat liver and kidney-cortex slices. Biochem J. 1969 May;112(4):449–453. doi: 10.1042/bj1120449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFRIR E., GORIN E. Release of glycerol in conditions of fat mobilization and deposition. Metabolism. 1963 Jul;12:580–587. [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962 Nov;237:3354–3358. [PubMed] [Google Scholar]
- Zakim D. Effect of ethanol on hepatic acyl-coenzyme A metabolism. Arch Biochem Biophys. 1965 Aug;111(2):253–256. doi: 10.1016/0003-9861(65)90185-2. [DOI] [PubMed] [Google Scholar]


