Abstract
1. Dialysed extracts of rat costal cartilage were shown to possess an enzyme that hydrolyses inorganic pyrophosphate. 2. Inorganic pyrophosphatase activity assayed in the presence of 2mm substrate was maximal at pH6·8. 3. Mg2+ was essential for activity, which was greatest with 10mm or higher concentrations of Mg2+. 4. Extracts prepared from cartilage taken from suckling rats (<20g.) showed little or no hydrolytic activity, but as rat weight increased inorganic pyrophosphatase activity was detected, increased to a maximum in tissue from animals weighing about 40g., and then rapidly declined. 5. The increase in inorganic pyrophosphatase activity was associated with an increase in the uptake of 45Ca by the cartilage in vivo. 6. Accumulation of calcium, inorganic phosphate and magnesium occurred when inorganic pyrophosphatase activity was at its maximum. 7. Alkaline phosphatase activity, measured in the same extracts used to determine pyrophosphatase activity, was highest in the tissues of the animals weighing <20g., and decreased as inorganic pyrophosphatase activity increased to its maximum. 8. There was no direct relationship between alkaline phosphatase activity and the onset of calcification.
Full text
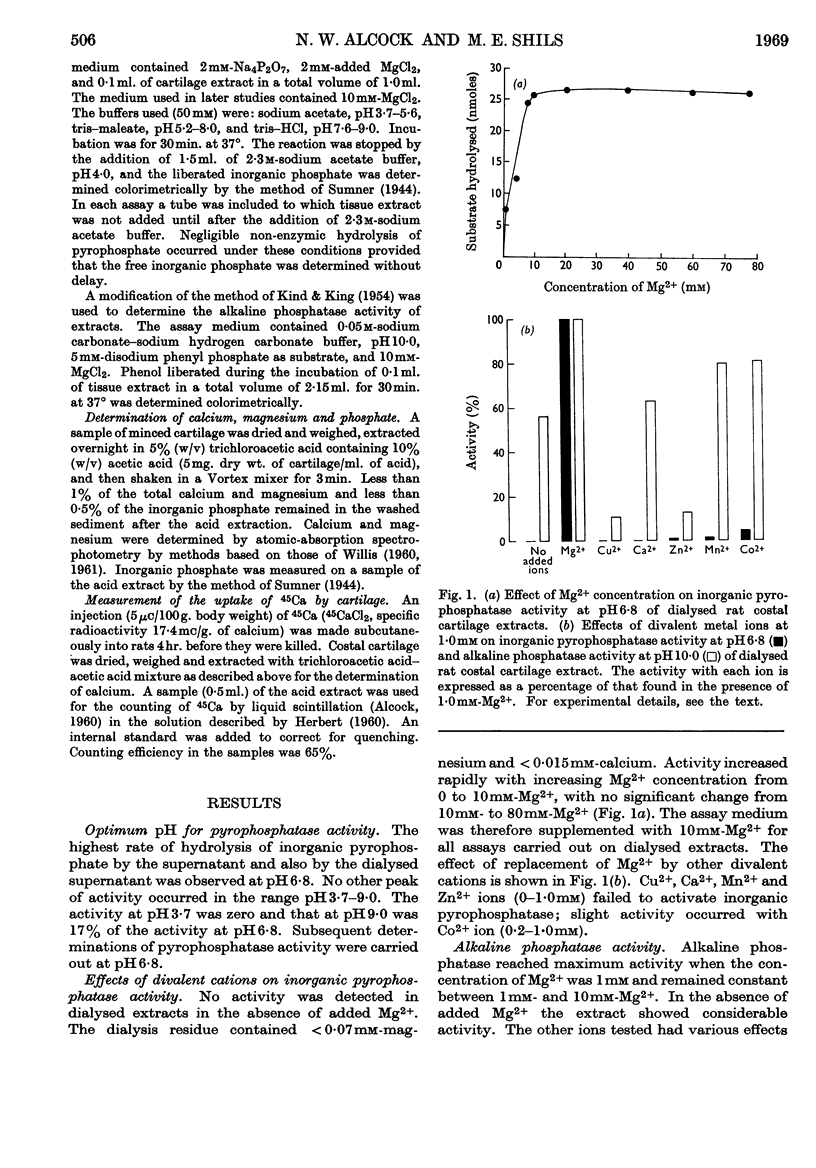
PDF
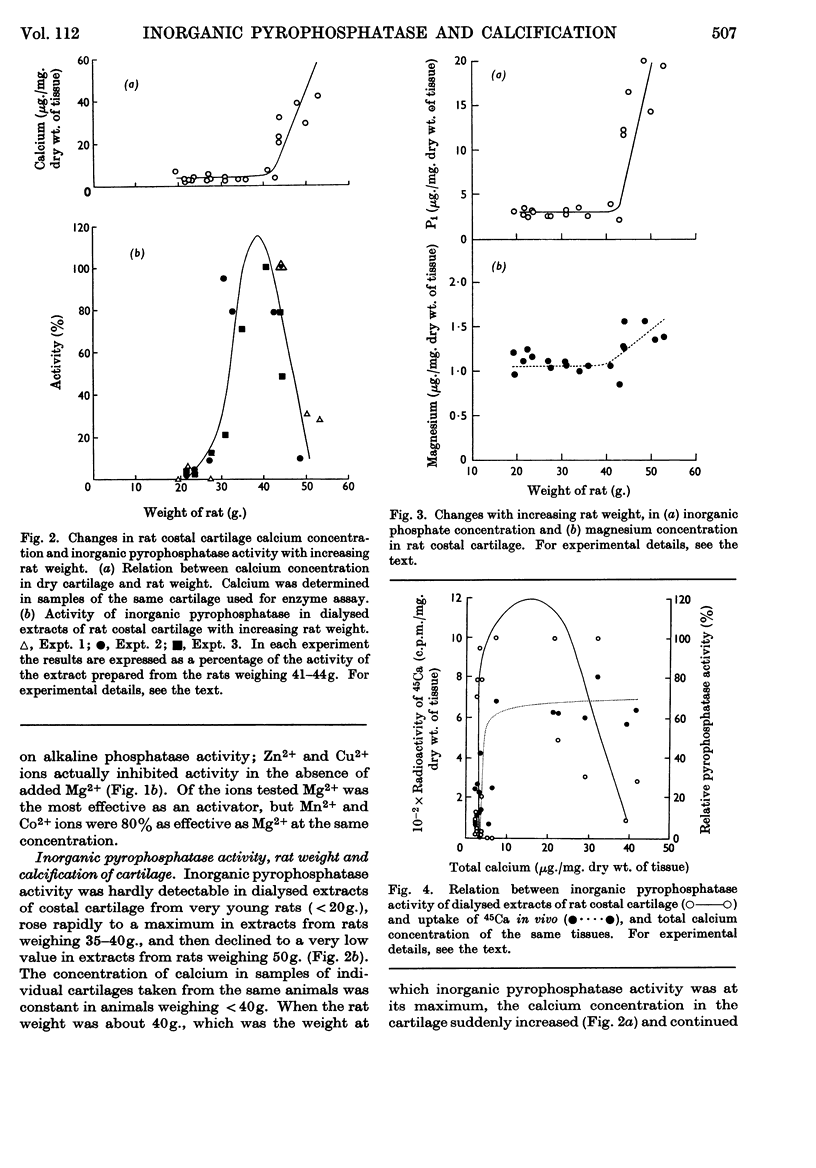



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH-FRANKENTHAL L. The role of magnesium in the hydrolysis of sodium pyrophosphate by inorganic pyrophosphatase. Biochem J. 1954 May;57(1):87–92. doi: 10.1042/bj0570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K., Webb E. C. Purification and properties of yeast pyrophosphatase. Biochem J. 1944;38(5):394–398. doi: 10.1042/bj0380394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTIER P., PICARD J. La minéralisation du cartilage ossifiable. I. La minéralisation du cartilage in vitro. Bull Soc Chim Biol (Paris) 1955;37(4):485–494. [PubMed] [Google Scholar]
- CARTIER P., PICARD J. La minéralisation du cartilage ossifiable. II. Le système ATPasique du cartilage. Bull Soc Chim Biol (Paris) 1955;37(5-6):661–675. [PubMed] [Google Scholar]
- CARTIER P., PICARD J. La minéralisation du cartilage ossifiable. III. Le mécanisme de la réactón ATPasique du cartilage. Bull Soc Chim Biol (Paris) 1955;37(11):1159–1168. [PubMed] [Google Scholar]
- Cox R. P., Gilbert P., Jr, Griffin M. J. Alkaline inorganic pyrophosphatase activity of mammalian-cell alkaline phosphatase. Biochem J. 1967 Oct;105(1):155–161. doi: 10.1042/bj1050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. H., Moss D. W. Inhibition of the orthophosphatase and pyrophosphatase activities of human alkaline-phosphatase preparations. Biochem J. 1967 Mar;102(3):917–921. doi: 10.1042/bj1020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 1962 Sep 1;195:911–911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- FLEISH H., NEUMAN W. F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961 Jun;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- Fleisch H., Russell R. G., Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966 Nov 26;212(5065):901–903. doi: 10.1038/212901a0. [DOI] [PubMed] [Google Scholar]
- Fleisch H., Schibler D., Maerki J., Frossard I. Inhibition of aortic calcification by means of pyrophosphate and polyphosphates. Nature. 1965 Sep 18;207(5003):1300–1301. doi: 10.1038/2071300b0. [DOI] [PubMed] [Google Scholar]
- Jibril A. O. Phosphates and phosphatases in preosseous cartilage. Biochim Biophys Acta. 1967 Aug 29;141(3):605–613. doi: 10.1016/0304-4165(67)90189-4. [DOI] [PubMed] [Google Scholar]
- KIND P. R., KING E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954 Nov;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay H. D. The phosphatases of mammalian tissues: Pyrophosphatase. Biochem J. 1928;22(6):1446–1448. doi: 10.1042/bj0221446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. W., Eaton R. H., Smith J. K., Whitby L. G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem J. 1967 Jan;102(1):53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R., WALKER P. G. The occurrence of pyrophosphate in bone. J Bone Joint Surg Br. 1958 May;40-B(2):333–339. doi: 10.1302/0301-620X.40B2.333. [DOI] [PubMed] [Google Scholar]
- Pynes G. D., Younathan E. S. Purification and some properties of inorganic pyrophosphatase from human erythrocytes. J Biol Chem. 1967 May 10;242(9):2119–2123. [PubMed] [Google Scholar]
- Robison R., Rosenheim A. H. Calcification of hypertrophic cartilage in vitro. Biochem J. 1934;28(2):684–698.1. doi: 10.1042/bj0280684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R. The Possible Significance of Hexosephosphoric Esters in Ossification. Biochem J. 1923;17(2):286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Adams J. M., Jr Localization mechanism of calcification in transplants of aorta. Ann Surg. 1967 Jul;166(1):1–18. doi: 10.1097/00000658-196707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]


